Formation and Structure of Alkanoates (Esters)
Alkanoic acids react with alkanols to form products called alkanoates (generally called esters) and water. The reaction is known as esterification reaction.
Example: The reaction between ethanoic acid and ethanol.
CH3COOH +
ethanoic acid |
C2H5OH
ethanol |
 |
CH3COOC2H5
ethyl ethanoate |
+ H2O
water |
As expressed above, the reaction involves the loss of hydroxyl ion - OH- by the acid, while the alkanol loses a hydrogen ion, H+ to form the products.
Therefore, the structural formula of esters of monocarboxylic acids is ROORI or
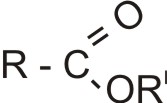
Where R is the alkyl group of the acid; RI is the alkyl group of the alkanol - R and RI may be same or different.
Nomenclature of Esters
Esters or alkanoates are read as alkanoates. The -ioc of the acid is replaced with -oate, and the alkyl group of the alkanol is read first.
Example: The ester CH3COOC2H5 is read as ethyl (from ethanol) ethanoate (from ethanoic acid).
Other examples: Alkanoates from ethanoic acid and methanol:
CH3COOH +
|
CH3OH
|

|
CH3COOCH3
methyl ethanoate |
+ H2O
water |
The ester formed is methyl (from methanol) ethanoate (from ethanoic acid).
Akanoates from propanoic acid and ethanol:
CH3CH2COOH +
|
C2H5OH
|

|
CH3CH2COOC2H5
ethyl propanoate |
+ H2O
water |
The ester is read as ethyl (from ethanol) propanoate (from propanoic acid).
Note: The reaction between alkanoic acids and alkanols to form alkanoates (esters) is called esterification and not neutralization.
Neutralization involves ionic reactions between acids and bases to form salts and water only (they are usually very rapid).
Esterification reactions are reversible and involve covalent molecules, and are usually very slow - they are aided by adding some conc. mineral acids as catalyst (this helps to absorb the water formed, thereby preventing the equilibrium from moving backward).
Esters occur naturally as fats and oils in plants and animals. Many of them have pleasant smells which produce the fragrance of flowers and the flavors of fruits.
Fats and Oil as Alkanoates
Fats and fatty oils are esters of long-chain carboxylic acids (fatty acids) and a trihydroxy alkanol (propan-1,2,3- triol) commonly called glycerol.
Glycerol is a viscous, non-toxic liquid, very miscible with water and of high boiling point (due to high degree of hydrogen bonding).
It has been established that all fats and fatty oils are made up of the same alkanol (propan -1,2,3- triol- glycerol) while the carboxylic acid component may differ - some may be saturated while others may be unsaturated.
Hence, fats and oils are also regarded as glycerides of fatty acids. Both the fatty acids and glycerol are combined by condensation reaction - with the elimination of water molecules.
The simplest combination is 3 molecules of fatty acids and 1 molecule of glycerol to give 1 molecule of fat or oil and 3 molecules of water.
Where R, RI and RII may be same or different. On hydrolysis, a particular fat may produce several different acids which made it up, but the same alkanol (i.e. glycerol).
The following are the common fatty acids found in all fats and many fatty oils:
1. Octadecanoic acids (commonly called stearic acid) - C17H35COOH
2. hexadecanoic acid (commonly called
palmitic acid) - C15H31COOH
3. octadec - 9 - enoic acid (commonly called oleic acid) - C17H33COOH
4. octadeca -9, 12-dienoic acid (commonly called linoleic acid) - C17H31COOH
Of the above, oleic and linoleic acids are unsaturated.
Fats are the solid glycerides which are composed predominantly of saturated fatty acids. Fatty oils are the liquid glycerides which are composed mainly of unsaturated fatty acids.
The term ‘fatty oils’ is used to distinguish glycerides from other oils, such as mineral oils and essential oils which are not glycerides.
Hydrogenation of Oils - Margarine Production
In the presence of finely divided nickel as catalyst, an oil can be converted to a fat by passing hydrogen into it and heating it to about 180oC, and about 5 atm pressure. This process is employed in the preparation of margarine using vegetable oils such as palm oil, groundnut oil and soya-bean oil.
The reaction is actually the saturation of the unsaturated fatty acids of the oil by hydrogen. The oil to be used is first treated with activated charcoal (heated charcoal) to decolorize it
Uses of Fats and Oils
1. In cooking - e.g. groundnut oil, palm oil and corn oil.
2. For the production for soaps, glycerine,
margarine and glycerine drugs.
.