|
Home
Chemical Equation
A chemical equation is the symbolic representation of a chemical reaction. It shows the chemical formulae of the reactants and the products, the mole ratios by which the reactants combine, as well as the mole ratios by which the products are formed. It shows the direction of the reaction; the state of matter in which the reactants and products are present.
For example, the chemical reaction between aqueous solution of hydrochloric acid and aqueous solution of sodium hydroxide that leads to the formation of sodium chloride and water can be expressed by the equation:
HCl(aq) + NaOH(aq) → NaCl(aq)
+ H2O(l)
In the above equation, the reactants (hydrochloric acid and sodium hydroxide) and the products of the reaction (sodium chloride and water) are shown by their formulae HCl, NaOH, NaCl and H2O respectively.
The mole ratio of the reactants and the products, which is the stoichiometry of the reaction is also expressed as 1:1:1:1. The direction of the reaction is shown by the forward arrow, which means that the reaction goes in one direction to produce sodium chloride and water.
Note that there are some reactions that can proceed both in the forward and backward direction in the same system. Such reactions are called reversible reactions and two half arrows to either side are used.
Example of reversible reaction is
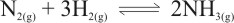
Also in the hydrochloric acid and sodium hydroxide reaction above, the state or phase of the reactants and the products are indicated in the chemical equation. HCl, NaOH and NaCl are aqueous solutions (the substances are in solutions in water) while H2O is in liquid state.
Chemical equations are useful in the following ways: To deduce quantities of reactants needed for reactions, or quantities of products expected from chemical reactions. To deduce quantities of energy involved in chemical processes. To give vivid explanations of chemical reactions.
Balancing of Chemical Equations
For a chemical equation to be useful, it must be balanced. A balanced chemical equation means that all the elements represented in the equation must have equal number of atoms on both the left and the right sides of the equation.
For example, in the chemical equation N2(g) + 3H2(g)
→ 2NH3(g) there are two elements, N and H. N has two atoms on the left where it is in a molecule of nitrogen gas and on the right hand side it also has two atoms where it is in 2 molecules of ammonia (NH3) gas. H is having six atoms on the left where it is in 3 molecules of hydrogen gas and on the right it also has six atoms in 2 molecules of ammonia gas.
Since the elements N and H have the same number of atoms on both sides, the chemical equation is therefore balanced. A balanced chemical equation shows that matter is neither created nor destroyed during chemical reactions, but change from one form to another. It therefore satisfied the law of conservation of matter.
Exercise: Balance the chemical equations
(a). P4O6 + O2 → P4O10 ; (b). HClO
→ HCl + O2 ; (c). NH4NO3 → N2O + H2O
(a). P4O6 + O2 → P4O10
The equation contains two elements, phosphorus (P) and oxygen (O). P has four atoms on both sides. It is therefore balanced.
Oxygen has a total of eight atoms on the left side and ten atoms on the right and is therefore not yet balanced. To balance the oxygen, put 2 in front of O2 on the left hand side. This brings the total number of oxygen atoms on the left hand side to be ten, same as those on the right side of the equation.
P4O6 + 2O2 → P4O10
The chemical equation is therefore balanced.
(b). HClO → HCl + O2
The equation has three elements – hydrogen (H), chlorine (Cl) and oxygen (O). Hint: first, identify the element that is alone in a molecule and balance it. Oxygen is alone in the molecule O2 on the right side. It has two atoms while on the left side it has one atom. It therefore needs to be balanced by having a 2 in front of HClO.
The equation becomes 2HClO → HCl + O2
This is not yet balanced as H and Cl have 2 atoms each on the left side while on the right side they have one atom. To balance them, we need to put 2 in front of HCl on the right side. The equation becomes 2HClO
→ 2HCl + O2 and is now balanced with all the elements having the same number of atoms on both left and right hand side.
(C). NH4NO3 → N2O +H2O
There are three element to balance in the equation: N, H and O. Nitrogen has two atoms on the left side and also two atoms on the right, making it balanced. For hydrogen, there are four atoms on the left side and two atoms on the right. Hydrogen needs to be balanced by placing 2 in front of H2O on the right side.
The equation becomes NH4NO3 → N2O +2H2O
Balancing hydrogen has also resulted in the balance of oxygen atoms – three atoms of oxygen are both on the left and right side (one in N2O molecule and two in H2O molecule). The chemical equation is therefore completely balanced.
|