|
Home
Electrode Potential
When a metal rod is dipped into a solution of its salt, the reduced and
oxidized forms of the metal come into contact.
For example, a zinc rod dipped into a solution of zinc sulphate – there are two possible tendencies:
1. For zinc ions in solution near the rod to draw electrons from the natural zinc atom in the rod and become reduced and deposited as neutral zinc atoms.
I.e. Zn2+(aq) + 2e-(aq) → Zn(s) – reduction.
2. For neutral zinc atom in the rod to go into solution as zinc ions, each leaving two electrons behind on the metal rod.
I.e. Zn(s) → Zn2+(aq) + 2e-(aq) – oxidation
Due to the above possible tendencies, there will exist a double layer of positive zinc ions and electrons on the rod.
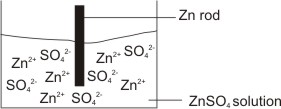
A potential will exist across this layer- this is called electrode potential.
Note: the tendency for a neutral metal to go into solution as ions depends on the relative ease with which electrons can be removed from it (ionization potential) and on the available energy when these ions are hydrated (hydration energy).
The more electropositive a metal is (example: Zn, with high ionization potential), the greater the tendency to go into solution as ions – oxidation.
The solution becomes more concentrated with the positive ions, and the electrode becomes more negative compared with the solution because electrons are deposited on it.
On the other hand, a less electropositive metal (example, copper) in the solution of its salt, will have its ions more likely to acquire electrons from its rod and become reduced – rather than being oxidized.
Therefore, the concentration of Cu2+ in solution is less, and the concentration of electrons on the rod becomes less compared with the solution.
Standard Electrode Potential
To measure the standard electrode potential, EƟ of a metal in its salt solution (1 M) standard hydrogen electrode (at standard conditions
– 1 M ion concentration, temperature of
298 K and a pressure of
1.013 x 105 N M-2) is coupled with it to form
an electrochemical cell.
Example, to determine the standard electrode potential, EƟ of zinc immersed in a solution
of 1 Molar Zn2+. This is coupled with the standard hydrogen electrode.
Note: standard hydrogen electrode is composed of pure hydrogen at 1.013 x 105 N M-2 (standard pressure) passed over platinized platinum dipped into a molar solution of H+(aq).
When the two electrodes are connected by a wire, a spontaneous reaction occurs and electrical energy is generated. The following reactions occur at the different electrodes: hydrogen been lower in the electrochemical series – reduction would occur at the standard hydrogen electrode, i.e. electrons flow from the zinc electrode (anode – due to excess electrons on the zinc rod) to the hydrogen electrode (cathode).
The half-cell reactions are:
Zn(s) → Zn2+(aq)+ 2e-(aq) – oxidation (Anode)
2H+(aq) + 2e-(aq) → H2(g) – reduction (Cathode)
Net reaction: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Both electrodes are standard. The measured e. m. f. is the sum of the standard electrode potentials of the two electrodes.
Note: the standard electrode potential of hydrogen electrode is zero.
Hence, the measured e. m. f. of the cell is also the standard electrode potential, EƟ of the standard electrode of the metal in its salt solution (i.e. of Zn in 1 molar Zn2+ solution).
By convention, a +ve sign is assigned to the EƟ value if the electrode is that of a metal which is more reduced than hydrogen, or which is less oxidized than hydrogen.
A –ve sign denotes EƟ for electrode of a metal which is less reduced than hydrogen, or which is more oxidized than hydrogen.
Therefore, EƟ is based on reduction, and the standard EƟ of Zn/Zn2+(1M) electrode is
-0.76 volt.
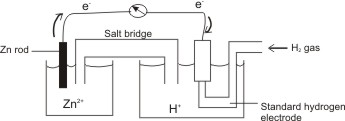
Cell of zinc electrode coupled with hydrogen electrode.
Note: The cell is represented as Zn(s)/Zn2+(aq) (1 M) // 2H+(aq)(1 M)/ H2(g)
Anode (oxidation) Cathode (reduction)
Electrons flow from the anode to the cathode
|