|
Home
Gas Laws
To understand gas laws, you
first need to understand the basic characteristics of gases. These include the
following facts:
- Gases are easily compressible – i.e., in the gaseous state, matter is not compact.
- Gases exert pressure.
- Gases are highly expansible.
- Gases diffuse completely into one another, so that any mixture of gases is homogeneous.
Note: the parameters used in describing gases are volume; pressure and temperature.
A change in the temperature or pressure will affect the volume.
Boyle’s Law
Boyle’s law states that the volume of a given mass of a gas is inversely proportional to the pressure at constant temperature.
What the law is simply saying is that at constant temperature, doubly the pressure of a given mass of gas, the volume is decreased by half.
I.e.
V
α
1/P ;
V = k/P
PV = k (the product of P and V is a
constant).
lf V1 is the original or initial volume of the gas at pressure P1, and V2 the volume at a final pressure P2, then
V1 / V2 = P2
/ P1
or
P1V1 = P2V2
Boyle’s law is illustrated by the P – V curve below:
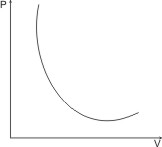
Note: Boyle’s law is not obeyed at low temperatures and high pressures (experiments show that PV Product is not constant at these conditions).
Calculation Based on Boyle’s Law:
A flask is made of two vessels A (100 cm3 capacity) and B (300 cm3 capacity) separated by a tap. A was
filled with a gas at 748 KNM-2 pressure and B was evacuated until the pressure inside it was negligible. Assuming that the temperature remains constant, calculate the pressure in the flask when the tap is opened, allowing gas to flow from A to B.
Solution:
V1/V2 = P2/P1
Where V1 = 100 cm3, V2 = sum of volumes A and B = 400 cm3, P1 = 748 KNM-2, V1 = ?
100/400 = P2/748
P2 = 748 x 100/400 = 187 KNM-2
Note: The initial pressure is due only to the effect of gas A, thus initial volume is that of only A as well.
You don’t have to convert volume to m3, leave it in whatever unit it is given.
Charles’ Law
Charles’s law states that, at constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature. This means that if the temperature of a gas is doubled, its volume is doubled as well, and if the temperature is halved, its volume is also halved.
I.e.,
V
α T
;
V = kT ;
k= V/T
If V1 is the volume of a fixed mass of a gas at the absolute temperature T1, and V2 is volume at temperature T2,
then:
V1/TI = V2/T2 = k
The figure below illustrates
Charles’ law.
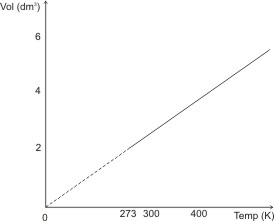
or
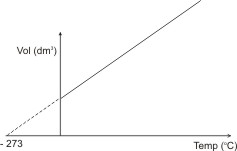
If a gas were to follow the above relationship indefinitely upon cooling, its volume would be reduced to 0 (zero) at -273 oC (or 0 oK). Actually, all gases have been found to liquefy before this temperature is reached. This temperature is called the absolute zero.
It is the temperature (-273 oC or 0 oK) whereby
theoretically, gases become liquid.
Note:
Charles’ law, like Boyle’s law is not obeyed at low temperatures and high pressures - the observed volume is less than that predicted by Charles’ law.
Hence, both Charles’ and Boyle’s laws only
apply to the behavior of an ideal or perfect gas.
Calculation Based on Charles'
Law
A fixed mass of gas has a volume of 200 cm3 at 27oC, calculate the volume when the temperature is increased to 87oC and the pressure kept constant.
Solution:
V1/TI = V2/T2
Where V1 = 200 cm3, V2 = ?, T1 = (273 + 27 = 300 K) , T2 = (273 + 87 = 360 K)
200/300 = V2/360
V2 = 360 X 200 /300
= 2 X 120 = 240 cm3
Combined Gas Law
A combination of Boyle’s and Charles’ laws gives the combined gas law.
I.e.
P1V1/T1 = P2V2/T2 = k
This can be used to calculate the temperature, pressure or volume of a given mass of a gas under different conditions.
Calculation Based on Combined
Gas Law
A given mass of a gas has a volume of 7.5 dm3 at 250 K and 80 K N M-2. Calculate the pressure at which it will have a volume of 7.2 dm3 at 270 K.
Solution:
P1V1/T1 = P2V2/T2
Where P1 = 80 KNM-2, P2 = ?, V1 = 7.5 dm3, V2 = 7.2 dm3, T1 = 250 K, T2 = 270 K
80 X 7.5 / 250 = P2 x 7.2 / 270
P2 = 270 x 80 x 7.5 / 250 x 7.2 = 90 KNM-2
|