|
Home
Hydrogen
The Two Forms of Molecular Hydrogen
A hydrogen molecule consists of two nuclei and two electrons, each of which is
spinning on its own axis.
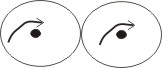
orthohydrogen
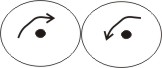
parahydrogen
According to the electronic theory of chemical bonding, the electrons must spin in opposite directions, but the two nuclei can spin in either the same or opposite directions.
Two forms of hydrogen molecule have been observed:
1. Orthohydrogen molecule - in this molecule, the nuclei are spinning in the same direction.
2. Parahydrogen molecule - here, the nuclei are
spinning in opposite directions.
At ordinary temperature, the normal ratio of orthohydrogen to parahydrogen in ordinary liquid hydrogen is about 3:1.
As the temperature is reduced, the ratio changes, and there is a conversion of orthohydrogen to parahydrogen. The conversion is exothermic, and the heat that is given off causes liquid hydrogen to boil.
However, since liquid parahydrogen does not undergo a similar conversion, it is more easily shipped and stored than ordinary liquid hydrogen. To produce liquid parahydrogen from orthohydrogen without it boiling off, catalytic methods have been developed. Hence, liquid hydrogen for use as rocket fuel and other uses can be made available.
Isotopes of Hydrogen
Three isotopes of hydrogen atom have been discovered:
1. Normal hydrogen, also called protium, 11H - this is the most abundant isotope of the three. It consists of only a proton, hence it has a mass of 1 amu.
2. Deuterium (D), 21H - this consists of a proton and a neutron, with a mass of 2 amu. Although the nucleus of a deuterium atom is not one of the “fundamental” particles, it has been given the special name deuteron.
3. Tritium (T), 31H - this has a proton and two neutrons, making it mass 3 amu. Only a small amount of tritium occurs naturally because of its
radioactive instability. The fact that it exists at all is due to the action of cosmic rays.
For the fact that hydrogen is a very light element, the percentage difference in the masses of the above three isotopes is so significant that it causes them to exhibit appreciable different properties from one another (this is why they are given different names), unlike isotopes of heavy elements, in which the percentage difference in their masses is not enough to cause them to show appreciable different properties.
For the diatomic hydrogen molecule, three isotopic varieties are also found. These are: H2, HD and D2. At temperature approaching the boiling point, HD undergoes partial conversion to H2 and D2. The density of D2
gas is twice as great as that of H2 gas.
Related:
Production of Hydrogen
Properties and Uses of Hydrogen
|

