|
|
|
Home
Polymerization Reactions of The Alkenes
Polymerization can be defined as the binding of two or more
simple molecules (called monomers) to form large molecular compounds (called
polymer).
Several molecules of ethene can combine at high temperatures (about
200 oC) and
pressures (above 100 atm) in the presence of a trace of oxygen to form large or
complex molecular structure (polyethene), with the release of heat.
2n (CH2
= CH2)
®
(-CH2 -CH2 - CH2- CH2 -)n
Note:
This type of polymerization is addition
polymerization - the same monomer (ethene) links up with one another to form a
giant molecule without a gain or loss of material.
Hence, the molecular mass of
addition polymers is the same as the sum of the molecular masses of all monomers
that combined, i.e., it is the multiple of that of the monomer. nA
® (A)n
. Where A is the monomer and (A)n
is the polymer of the original compound, A.
Synthetic Rubber
Another example of polymerization reactions of the alkenes is
in the manufacture of synthetic rubber. A number of synthetic rubber have now
been developed so as to find a replacement for natural rubber. Natural rubber is
a polymer of 2-methyl buta -1,3 -diene (previously known as polyisoprene).
|
n(CH2=C(CH3) -CH = CH2)
2-methyl buta-1,3-diene (isoprene) |
® |
...-CH2 -C(CH3)=CH - CH2-... |
|
|
1. The most important synthetic rubber is styrene-butadiene. The process for the manufacture of this synthetic rubber involves
the warming of styrene (i.e. phenyl ethene) with buta-1,3-diene in water, in
the presence of an emulsifying agent and the reaction initiator.
|
n(CH2 = CH - C6H5) + n
(CH2= CH - CH = CH2)
styrene buta-1,3-diene |
® |
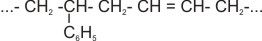
Polymer |
|
|
2. Thiokol: This is made by heating 1,2-dichloroethane,
ClCH2-CH2Cl
with sodium polysulphide, Na2Sx.
3. Neoprene rubber: This is a polymer of chloroprene (2-chloro-1,3-butadiene), CH3= CCl - CH = CH2
4. Buna S rubber: This is a co- polymer of 1,3 - butadiene
and styrene with soduim as catalyst. The name Buna S is an abbreviation for
butadiene - Na- styrene.
Vulcanization of Rubber
This is the addition of a
calculated amount of sulphur to rubber. The mixture is carefully heated and the
rubber becomes more tough, more elastic, less sticky, less soluble in organic
solvent, and insensitive to heat.
Notice that the sulphur acts to join up
adjacent molecular chains of the rubber. The industrial use of rubber is
possible only because of vulcanization.
Uses of Ethene
1. As a raw material for the manufacture of a wide range of
chemicals such as ethanol, ethane, epoxy ethane, and tetraethyl lead.
2. For the manufacture of polythene.
3. For the manufacture of styrene (i.e., phenylethene, C 6H5-
CH = CH2).
Styrene can be polymerized into a plastic product (polystyrene) or it can be co-polymerized with buta-1,3-diene to produce synthetic rubber.
4. Ethene can also be used for the production of 1,2-dichloroethane which is co-polymerized with sodium polysulphide to give a synthetic rubber.
5. Ethene at low concentrations can be used to quicken the ripening of
fruits.
Related:
Alkenes
Isomerism in Alkenes
|
|
|