|
Home
Separation of Mixtures
Components of mixtures are
usually separated by physical means (they are therefore purified). There are
different physical methods which can be employed to separate mixtures. The
particular technique chosen for any given mixture depends on the nature of the
constituents. Here are some separation techniques:
Evaporation
This method is used to
separate components of soluble solid/liquid mixtures and volatile/involatile
liquid mixtures. The principle governing this method is the fact that molecules
of liquid substances when they gain heat, become gaseous and are lost from the
surface. Notice that the liquid, haven vaporized is not collected but lost to
the atmosphere. The other component (which is required), is then collected.
Example – a mixture of sodium chloride and water.
Distillation
This is used to separate
components of liquid/liquid mixtures and soluble solid/liquid mixtures. It
involves heating the mixture, and the vapor formed is allowed to cool, liquefy
and is collected as pure liquid. Thus, each component of the mixture is
purified. The principle behind this method is based on the fact that when
liquids are heated to their boiling points, they become gaseous, and when the
gases are cooled, they change back to the liquid.
Note: While evaporation is mostly used for solid/liquid mixtures, distillation
is mostly used to separate liquid/liquid mixtures. Both evaporation and
distillation involve gain of heat, and then vaporization. In evaporation, the
vapor formed is allowed to escape into the atmosphere, while in distillation,
the vapor is not lost but cooled, liquefied and collected as pure liquid.
Distillation is used to purify solvents. There are two kinds of distillation –
simple and fractional distillation:
Simple Distillation: This is used to separate mixtures of volatile/involatile
liquids, or for mixtures of liquids whose boiling points are wide apart (by at
least 100oC). An example of such mixtures is the mixture of water and
ink.
Fractional Distillation:
This is used to separate a mixture of liquids whose boiling points are close
(boiling point difference of not more than 20-30oC). Examples of
mixtures that can be separated by this method include: petroleum; alcohol and
water; liquid air (a mixture of oxygen (b.pt 90 K), nitrogen (b.pt 77 K) and
water (b.pt 1000C)).
Sublimation
Sublimation is suitable for
solid mixtures containing solid substances that can vaporize directly when
heated. Examples of such substances are iodine crystals, ammonium chloride,
anhydrous aluminium chloride, anhydrous iron(III) chloride and benzoic acid. The
vapor is cooled away from the other component(s) and collected as solid.
Dissolution
The principle behind this
technique is that some solid substances are soluble in certain kind of
solvent, while others are not. Hence, it is used generally to separate soluble
substances from insoluble ones. For example, a mixture of sodium chloride
crystals and sand – the sodium chloride is soluble in water while sand is not.
Therefore, water is added to the mixture to dissolve sodium chloride while
leaving the sand to settle.
Note: organic solvents generally dissolve organic substances, e.g. kerosene
dissolves wax, grease, fats and oils. Inorganic solvents dissolve inorganic
substances, and ionic solvents dissolve ionic substances. Common solvents for
sulphur are: carbon(IV) sulphide, CS2 and methylbenzene (toluene).
Common solvents for iodine are: ether (ethoxyethane), alcohol, carbon
tetrachloride, CCl4 and potassium iodide.
Water soluble salts
includes: All common trioxonitrates(V) of metals. All common salts of sodium,
potassium and ammonium. All common tetraoxosulphates(VI), except: barium
tetraoxosulphate(VI) and lead(II) tetraoxosulphate(VI). Notice that calcium
tetraoxosulphate(VI) is sparingly soluble. All common chlorides except those of
silver, mercury(I) and lead.
Filtration
This is used to separate
liquid components of mixtures from the solid components (which are in
suspension). The principle of this technique is that the particles of liquid are
small enough to pass through the filter material while those of solids are not.
Notice that the solid particles are in suspension.
If they were settled at the
bottom, then the process would be decantation and not filtration. Decantation
does not involve the use of filter materials; it is the run-off of the liquid
component, leaving the solid behind. Decantation will come before filtration
(depending on whether the mixture contains solid components which are large and
heavy enough to settle).
Both filtration and
decantation usually follow the process of dissolution. E.g. after the sodium
chloride component of a mixture of sodium chloride and sand is dissolved in
water, the liquid component (sodium chloride solution) is decanted (separation
from sand), and then filtered to obtain clear sodium chloride solution.
Crystallization
The principle of this method
is based on the fact that soluble salts are only soluble to certain
concentrations at a given temperature. Decrease in the temperature of their
saturated solutions will see the salts forming out of the solution. It is used
to obtain a soluble salt from its solution, and it involves heating the solution
up to the point of saturation (for salts which crystallize with water of
crystallization, e.g., ZnSO4 . 7H2O).
Cooling the solution below
this point results in the formation of the crystals from the solution. For salts
which do not crystallize with water, e.g., NaCl, their solutions are heated to
dryness to produce them. Notice that salts which crystallize with water are not
heated to dryness, otherwise, their crystalline nature will be lost. To purify
further, the salt can be recrystallized. I.e., the crystals obtained is
dissolved in hot distill water and the process of crystallization is repeated.
Notice that crystallization
needs evaporation (by heating) for the solution to become saturated. It is
possible to separate a mixture of more than one water-soluble salt by
crystallization. This is because the solutions of different substances attain
saturation at different temperatures. A solution containing a mixture of
different substances therefore crystallizes its components separately when
cooled below the saturated points of the different components in solution - this
is known as fractional crystallization.
Chromatography
This method is mostly
popular for the separation of colored components of pigments (e.g. ink and
paints). However, it is useful also in separating certain non-colored components
of mixtures.
Note: All chromatographic methods involve two phases, namely: stationary phase
and mobile phase. Separation is based on the relative speed of the components of
the mixture in-between the two phases.
If the stationary phase is a solid, the process is called adsorption
chromatography. If the stationary phase is a liquid, the process is called
partition chromatography.
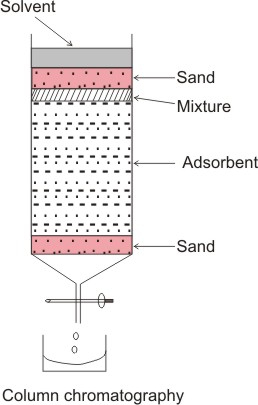
Column chromatography (adsorption chromatography). The stationary (adsorbent) is
a solid, e.g. finely divided alumina and silica gel. The column is usually a
glass tube with a tap at the bottom packed with the adsorbent and the mobile
phase (the eluting solvent).
As the solvent travels down
the column, it carries with it the different components, which travel down at
different rates depending on the extent to which they are adsorbed. More
strongly adsorbed components travel down more slowly than less adsorbed ones.
Hence, components are separated based on their different degree of adsorption on
to the stationary phase as they move down the column (which causes them to move
at different speeds).
Paper chromatography
(partition chromatography).
Paper chromatography, also
known as partition chromatography is a technique that involves the use of strips
of filter paper. Notice that the stationary phase in paper chromatography is the
moisture in the paper, and not the paper itself. This is an example of partition
chromatography. Separation depends on the different degree of motion (i.e.
speed) of the components of the mixture between the stationary water phase and
the mobile chromatographic solvent (due to the different affinity the components
have for both the stationary and mobile phases).
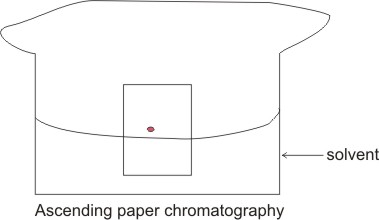
The material to be separated
is applied as a spot near the bottom of the strip of paper. It is dipped into
the solvent and the chromatogram left to develop. The solvent (e.g. propanone or
ethanol) ascend the strip of paper by capillary action, and carries the solute
along with it, different components travel at different rates depending on their
relative affinity for both the mobile and stationary phrases.
This is ascending paper
chromatography. A descending technique can be made by allowing the solvent to
flow down the strip from a tray containing the solvent. Components of mixtures
with greater affinity for the mobile phase than the stationary phase are
separated first.
Precipitation
Precipitation is used to
separate a salt which is soluble in one solvent, forming a mixture with that
solvent, but become insoluble when another liquid which mixes well with the
mixture but which does not dissolve the salt is added. The salt will therefore
be precipitated from the solution and collected by filtration. For example,
iron(II) tetraoxosulphate(VI) is soluble in water to form a mixture (i.e. a
solution). When ethanol is added to the solution (ethanol is miscible with
water), the iron(II) tetraoxosulphate(VI) will be precipitated from the solution
as it is insoluble in ethanol.
Sieving
Sieving is used to separate
solid mixtures whose components’ particle size differ greatly. A sieve is used
to make the separation. The particles of one component are small enough to pass
through the sieve, while those of the other are not, and are therefore held onto
the sieve, separated from the first. Notice that the principle of separation
used here is the large difference in the particle size of the components of the
mixture.
|