|
Home
The Electrochemical Series
The electrochemical series (see below) is an orderly arrangement of metals and their ions based on how well the ions accept electrons and become reduced.
The lower the position of metals and their ions in the series, the more likely their ions are reduced at the cathode, hence the more the preference for them been discharged.
Notice that during
electrolysis, reduction occurs at the cathode.
The Electrochemical Series
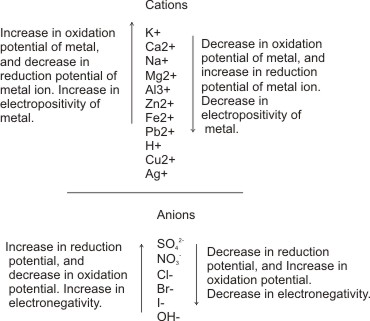
Example, in the presence of H+ and Na+ in a solution,
and if all other factors are constant, H+ will
accept electrons more readily and
subsequently be discharged at the cathode in
preference to Na+.
The electrochemical series also shows
the arrangement of negatively charged
particles which migrate to the anode. In the
same way as above, the lower the position of
a negatively charged particle in the series,
the more likely it gives off electrons and become
oxidized at the anode.
Example, considering all other
factors constant, a solution containing OH- and
SO42- ions will have the OH-, which is lower
in the electrochemical series discharged at the
anode in preference to SO42-.
|

