|
Home
The Lewis Concept of Acids and Bases
In 1923, the American chemist Gilbert Newton Lewis
proposed generalized definitions for acids that do not restrict them to
compounds containing hydrogen. Lewis defined an acid as any molecular or ionic
species that contains an atom capable of sharing a pair of electrons furnished
by a base. In the simplest terms, a Lewis acid is an electron acceptor.
The BrØnsted-Lowry concept is more general than the Arrhenius concept because it applies to both aqueous and non-aqueous solutions and also to reactions that occur in the absence of a solvent. Inspite of its breadth, the BrØnsted-Lowry concept is limited by its emphasis on the proton. Only compounds containing hydrogen can qualify as acids by the BrØnsted-Lowry definition.
In every instance of protolysis the BrØnsted-Lowry acid is the substance that donates the proton, whereas the Lewis acid is the proton itself. Examples:
| H+
+ |
 |
→ |
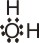 |
(H+
is the Lewis acid) |
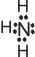 |
+
Ag+
+ |
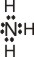 |
→ |
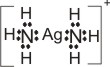 |
(Ag+
is the Lewis acid) |
 |
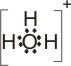
+ |
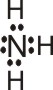 |
→ |
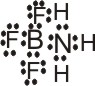 |
(BF3 is the Lewis acid) |
All positive ions are potential Lewis acids. Any molecule in which the central atom has an incomplete octet, e.g., BF3 or AlCl3, is a potential Lewis acid.
The Lewis Concept of Bases
Lewis defined a base as any molecular or ionic species that contains an atom having a pair of electrons available for sharing by an acid. In simplest terms, a Lewis base is an electron donor.
All negative ions are potential Lewis bases. Any molecule in which an atom has one or two unshared pairs of electrons, example, NH3 or H2O, is a potential Lewis base.
Examples of Lewis Acid-Base Reactions
Recall: Lewis acid - electron acceptor, Lewis base - electron donor
1.

Lewis
acid |
+ |
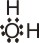
Lewis
base |
 |
 |
+ |
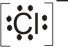 |
Instead of emphasizing that a proton is transferred to a water molecule, we can point to the fact that oxygen atom of the water molecule donates a pair of electrons to the proton and
thereby forms a coordinate covalent bond. In each of the following examples, the BrØnsted-Lowry base donates a pair of electrons to a proton and therefore acts as a Lewis base also:
2.

Lewis
acid |
+ |

Lewis
base |
 |
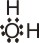 |
+ |
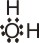 |
3.

Lewis
acid |
+ |

Lewis
base |
 |
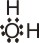 |
+ |
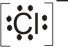 |
4.

Lewis
acid |
+ |
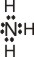
Lewis
base |
 |
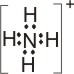 |
+ |
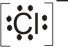 |
The above examples of Lewis acid-base reactions also conform to the BrØnsted-Lowry definition for acid-base reactions. The following examples are representative of the many Lewis acid-base reactions that do not conform to the BrØnsted-Lowry definition:
1. H+
+
Lewis
acid |
+ |

Lewis
base |
→ |
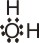 |
2. H+
Lewis
acid |
+ |
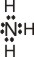
Lewis
base |
→ |
 |
3.

Lewis
acid |
+ |
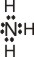
Lewis
base |
→ |
 |
4.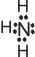
Lewis
base |
+
Ag+
+
Lewis
acid |
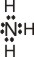
Lewis
base |
→ |
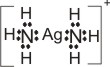 |
5.
Lewis
base |
+
Ag+
+
Lewis
acid |
Lewis
base |
→ |
 |
6.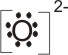
Lewis
base |
+ |

Lewis
acid |
→ |
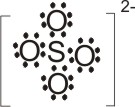 |
The compounds formed by the reaction of Lewis acids with Lewis bases are coordination compounds. Indeed, the fundamental acid-base reaction in the Lewis sense is the formation of a coordinate bond.
Those substances that are bases in the Lewis system are also bases in the BrØnsted-Lowry system, for electron donors can share electrons with any Lewis acid, including the proton.
Related Tutorials
The Concepts of Acids and Bases
What is Basicity of Acids
How to Determine Strength of Acids
Properties of Acids
Uses of Acids
Methods of Preparation of Acids
The BrØnsted-Lowry Concept of Acids And Bases
|

