|
Home
Alkanols, Ethanol
What is an alkanol?
Alkanols are compounds composed of the hydroxyl group (-OH). The -OH group is the functional group, i.e., the site of chemical reactions.
Most alkanols consist of one hydroxyl group - these are called monohydric alkanols. Others consist of more than one hydroxyl group - these are called polyhydric alkanols.
Example: ethane 1, 2- diol (also called ethylene glycol).
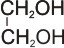
ethan-1,2-diol (glycol)
|
and |
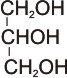
propan-1,2,3-triol (glycerol)
|
|
|
The monohydric alkanols form a homologous series, in which all members conform to the molecular formula CnH2n
+ 1 OH or CnH2n +2O .
Alternatively, the alkyl group CnH2n+1 can be represented by R, hence, the molecular formula becomes ROH.
The first five members are:
n = 1 CH3OH methanol
n = 2 C2H5OH ethanol
n = 3 C3H7OH propanol
n = 4 C4H9OH butanol
n = 5 C5H11OH pentanol
Classification of Alkanols
Alkanols can be classified as primary, secondary or tertiary, according to the number of alkyl or aryl groups attached to the carbon which bears the -OH group.
Note: A primary alkanol has only one alkyl or aryl group attached to the carbon bearing the -OH; a secondary alkanol has two alkyl or ary groups attached and a tertiary alkanol has three.
i.e., Primary alkanol - CH3CH2CH2CH2OH
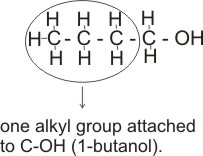
Secondary alkanol - CH3CH2CHOHCH3
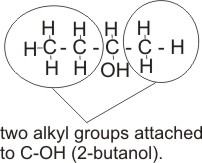
Tertiary alkanol - C6H5C(CH3)OHCH3
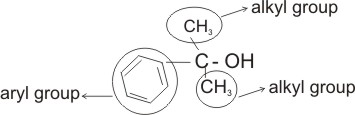
Two alkyl groups and one aryl group are
attached to C-OH (2-phenylpropan- 2-ol).
Production of Ethanol
There are various ways of making ethanol, they include:
(1). By fermentation
The important reaction in the production of ethanol by fermentation is the catalytic conversion of the sugar (glucose) into alcohol by the enzyme zymase, which is found in yeast.
The sugar is usually obtained from cheap sources that may be available.
E.g. i. From starchy food stuffs like rice, potatoes and barley: the starchy food stuffs are pressure- cooked to release the starch granules and then treated with malt (partially sprouted barley) for about an hour at 60oC.
The malt supplies an enzyme, diastase which hydrolyses the starch to sugar (maltose).
2C6H10O5(aq)
Starch |
+ |
H2O(l)
|
diastase → |
C12H22O11(aq)
maltose |
Yeast is added at room temperature- yeast contains two enzymes - maltase and zymase. Maltase hydrolyses maltose to glucose.
| C12H22O11(aq)
|
+ |
H2O(l)
|
moltase → |
2C6H12O6(aq) |
Zymase catalyses the decomposition of glucose to ethanol and carbon(IV) oxide.
| C6H12O6(aq)
|
zymase → |
|
2C2H5OH(aq) |
+ CO2(g) |
ii. From fruits, fresh palm wine (in some parts of Africa) and honey - these materials contain rich supply of sugars. The sugars are then fermented to alkanol by the addition of yeast.
Palmwine already contains yeast, which contains enzymes that hydrolyse the sugar to glucose and ferment the glucose to alcohol.
Where wood is readily available, saw-dust can also be used. The sawdust (cellulose) is first converted by hydrolysis to glucose by heating it with mineral acids under pressure (acid hydrolysis).
(C6H10O5)n
cellulose |
nH2O → hydrolysis |
|
nC6H12O6
glucose |
|
The glucose is then fermented (by the addition of yeast) into ethanol and carbon(IV) oxide.
Note:
* The process of converting a complex]
carbohydrate (e.g. starch) to a simpler carbohydrate (e.g. glucose) is called
hydrolysis.
* The breaking down of a complex
material such as starch into much simpler substances, such as ethanol and carbon(IV) oxide is known as
degradation.
Distillation of ethanol - the solution of ethanol obtained directly from fermentation is called ‘wash’. It contains low percentage of ethanol (less than 11%).
It is converted to solutions of higher percentage of ethanol by the process of fractional distillation - the boiling point of ethanol is 78oC, distillation is stopped at the required concentration.
For example, to make wines and beers, the solution (‘wash’) is distilled to a concentration of 8 to 20%; 20-50% for spirits such as whisky and gin.
The purest form of ethanol sold contains 95% ethanol - it is called rectified spirit. However, a purer form of ethanol can be obtained from rectified spirit by distilling it over quicklime (a dehydrant) - the concentration is 99.5% ethanol and is known as absolute ethanol - absolute ethanol is very hygroscopic.
Note: Fermented and distilled products are known as spirits.
(2). From petroleum products
One of the by-products from the cracking of crude oil fractions is ethene. Ethene can be converted to ethanol by absorbing it under pressure in conc. H2SO4 at 80oC.
Ethyl hydrogen tetraoxosulphate(VI) is formed and is diluted with water and distilled - ethanol is produced.
| C2H4(g)
+ |
H2SO4(aq)
® |
|
C2H5HSO4(aq)
Ethyl hydrogen tetraoxosulphate(VI) |
|
| C2H5HSO4(aq)
+ H2O(l) ® |
C2H5OH
Ethanol |
+
H2SO4(aq) |
|
|
Or, by a newly developed method of passing ethene and steam over sulphuric or phosphoric(V) acid as a catalyst at 600oC and 60 to 100 atm. pressure - ethanol is produced.
| C2H4(g)
+ H2O(g) ® |
C2H5OH(l)
Ethanol |
|
|
|
Properties of Alkanols
Physical properties:
1. The simpler alkanols, e.g. methanol and ethanol are liquids at ordinary temperature and their densities increase with number of carbon atoms. Solid members are found for alkanols of large carbon chain.
2. They are colorless, volatile and possess a characteristic smell.
3. They are soluble in water- due to the presence of hydrogen bonding with water molecules. However, their
solubility decrease with
increase in carbon atoms per molecule.
4. They have high boiling and melting points than the corresponding
alkanes -
due partly to increase in molecular weight and the presence of hydrogen bonding.
5. They have no action on litmus (they are neutral, i.e., they are neither acidic nor basic) .
Chemical reactions:
1. Reactions of the hydroxyl group
The functional group of the alkanols is -OH. Hence, the following characteristic reactions of alkanols occur at the - OH site.
Ethanol is used as a representative of the alkanols to illustrate their chemical properties:
(a). With sodium - sodium metal reacts vigorously at room temperature with ethanol. Hydrogen is liberated and sodium ethoxide (white deliquescent solid) is formed.
2C2H5OH(l) + 2Na(s)
®
H2(g) + 2C2H5ONa(s)
(b). With a chloride of phosphorus - phosphorus(V) chloride reacts vigorously with ethanol at room temperature. Steamy fumes of HCl and chloroethane are produced.
C2H5OH(l) + PCl5(l)
®
C2H5Cl(g) + POCl3(l) + HCl(g)
Note:
*The reaction with PCl5 is the
standard test for the hydroxyl group (-OH) in a straight chain organic com-
pound. The reaction is the replacement of -OH by chlorine.
*All members of the alkanol homologous series give the above reactions a and b, however, with decreasing degree as the number of carbon
atoms increases.
2. Reaction with conc. H2SO4 - ethanol reacts readily with conc. H2SO4 in two distinct ways, depending on the quantity of both acid and ethanol in reaction, and the temperature of reaction:
In excess of conc. H2SO4
and at high temperatures (above 170oC) - the reaction produces ethyl hydrogen tetraoxosulphate(VI) which decomposes to release ethene.
(a). C2H5OH(aq) + H2SO4(aq)
®
C2H5HSO4(aq) + H2O(l)
(b). C2H5HSO4(aq)
®
C2H4(g) + H2SO4(aq)
The mechanism of the reaction is thus:
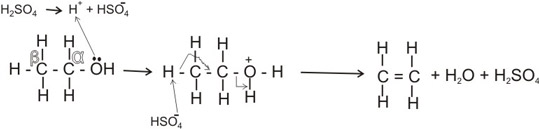
Note:
*The carbon bearing the -OH is the alpha (a) carbon, the next is beta (b).
During dehydration of ethanol, a hydrogen atom is lost from the
b-carbon, while the -OH group is lost from the
a-carbon.
*At lower temperatures and presence of excess ethanol, the product formed is ethoxyethane (or diethylether).
E.g.
(a). C2H5OH(aq) + H2SO4(aq)
® C2H5HSO4(aq)
+ H2O(l)
| (b). C2H5HSO4(aq) + C2H5OH(aq)
® |
C2H5OC2H5(aq)
Ethoxyethane |
+ H2SO4(aq) |
|
|
Note:
The above reactions are dehydration reactions because one mole of water is
released from both reactions to give ethene and ethoxyethane respectively.
Dehydration of 2-methyl-2-butanol:
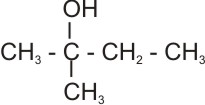
Here, hydrogen atom can be lost from either of the b carbon to produce two isomeric alkenes (i.e., internal and terminal alkenes) together.
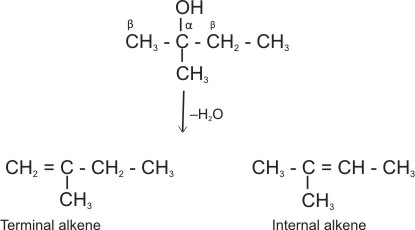
Internal alkenes are more stable, hence are produced more.
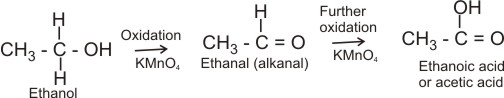
3. Oxidation - ethanol oxidizes in two stages with an oxidizing agent. E.g. when heated with acidified K2Cr2O7 solution, ethanol is oxidized to ethanal, while the potassium dichromate is reduced (its color changes from orange to green).
CH3CH2OH(aq)
® CH3CHO(g) + 2H+(aq) + 2e-(aq)
Further heating of more acidified K2Cr2O7 with the ethanal formed, produces ethanoic acid (acetic acid).
CH3CHO(g) + H2O(l)
® CH3COOH(aq) + 2H+(aq) + 2e-(aq)
Both stages of oxidation above can be achieved using catalyst. When ethanol vapor is passed over finely divided copper at 3000C, ethanal is formed.
When the ethanal vapor is passed over manganese(II) ethanoate, ethanoic acid is formed.
Note: the oxidation of ethanol in limited quantity of the oxidizing agent produces ethanal (partial oxidation); while with excess of the oxidizing agent, ethanoic acid is formed (complete oxidation).
This is the reason why wine (e.g. palm wine) sometimes become sour on exposure to air for a long time - the bacteria in it oxidize the ethanol to ethanoic acid.
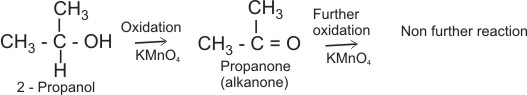
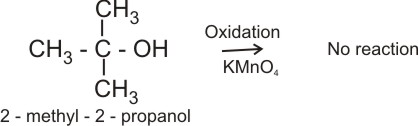
In general, primary alkanols are oxidized first to aldehyde and then to carboxylic acid; secondary alkanols are oxidized to ketones and tertiary alkanols are resistant to mild oxidation, but are degraded by strong oxidants like conc. HNO3 to mixtures of carboxylic acids and ketones.
Hence, by order of degree of oxidation, we have:
primary > secondary > tertiary alkanol.
Primary alkanol:
Secondary alkanol:
Tertiary alkanol:
4. Ester formation - ethanol reacts readily and reversibly with acids to form ethyl esters - this process is called esterification. Example, ethanol reacting with ethanoic acid:
C2H5OH(aq) + CH3COOH(aq)
®
ethanoic acid |
CH3COOC2H5(l)
ethyl ethanoate |
+ H2O(l) |
|
|
Combustion reaction - like every other organic compound, ethanol burns in oxygen or air with a pale-blue flame to produce water and carbon(IV) oxide.
C2H5OH(aq) + 3O2(g)
® 3H2O(l) + 2CO2(g)
Nomenclature of Alkanols
The I.U.P.A.C. naming of alkanols is similar to those of the aliphatic hydrocarbons discussed earlier. The numbering of the longest straight chain is dependent on the position of the -OH
group (it should occupy the lowest carbon position).
The -ane of a corresponding alkane is replaced with -ol for the alkanol.
The position of the carbon bearing the -OH is indicated in front of the parent name or in the middle of it, with hyphens separating it from the other part of the name.
Example:
(a).
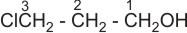
3- chloro -1- propanol or or 3- chloropropan -1- ol |
|
|
|
|
(b).
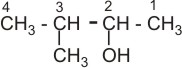
3- methyl -2- butanol
or 3- methyl butan -2- ol |
|
|
|
|
Uses of Ethanol
(1). As solvent for stains, polishes, resins, varnishes, lacquers, drugs, flavouring extracts, perfumes, soap and dyes.
(2). As fuel in racing cars and in rockets.
(3). In alcoholic beverages, e.g., beers, wines, and spirits.
(4). For the production of several important chemicals, e.g., ethanal, ethanoic acid, ethyl esters, ethoxyethane e.t.c.
(5). As an anti-freeze - due to its low freezing point of -117oC.
|

