The first and the simplest member
is when n is 1. i.e., CH4.
Homologous Series
A homologous series is a group of organic compounds which follow a regular
structural pattern, in which each successive member differs in composition by
the addition of a - CH2 group.The table below shows the first ten
alkanes and their formula:
| number of carbon atoms |
Formula |
Compound |
| n =1 |
CH4 |
Methane |
| n = 2 |
C2H6 |
Ethane |
| n = 3 |
C3H8 |
Propane |
| n = 4 |
C4H10 |
Butane |
| n = 5 |
C5H12 |
Pentane |
| n = 6 |
C6H14 |
Hexane |
| n = 7 |
C7H16 |
Heptane |
| n = 8 |
C8H18 |
Octane |
| n= 9 |
C9H20 |
Nonane |
| n = 10 |
C10H22 |
Decane |
Characteristics of Homologous Series:
1. There is a general molecular formula for all members of a particular
homologous series , for example, CnH2n+2 for the alkanes.
2. The molecular
formula of each member differs from the next by CH2.
3. Members of a homologous series possess similar chemical properties (as a
result of their similar molecular structures), though to different degrees.
Example,
the chemical reactions of the alkanes include substitution and combustion.
4. Members have different physical properties. For the alkanes, the first four
members are gases at ordinary temperature and pressure, while the next five to
ten members are liquids.
As the number of carbon atoms increases, solid members
are obtained (e.g. C20H42). The boiling and melting points increase with
increase in carbon atoms.
Although the alkanes are sparingly soluble in water,
their solubility decreases with increase in carbon atoms per molecule.
5. General methods of preparation are known, and can be applied to any member of
the series.
6. The names of all members have similar endings. Example, alkanes end with -ane.
7. There is the possibility of each member having its atoms arranged differently
to form different structures called isomers. As the number of carbon atoms
increases, the number of possible arrangement of those atoms increases rapidly.
Example, for the alkanes, there are 2 isomeric butanes, 3 pentanes, and 5 hexanes.
Structure of Alkanes
In alkanes, example, methane, carbon uses sp3 hybridization,
that is, the one 2s
orbital is mixed with the three 2p orbitals to form four atomic orbitals of
equal energies (i.e. four hybridized orbitals).
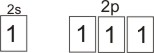
Hybridized orbitals
(sp3) |
|
|
|
|
The four sp3 orbitals then combine separately with the s orbital of four
hydrogen atoms to form methane, CH4.
Here, each of the bonds between carbon and
hydrogen is sp3 - s and is called sigma bond (σ) (this is the bond between two
hybridized orbitals, or between a hybridized orbital and an s orbital).
Note: all available orbitals in the valence shell of carbon are used, hence, the
compound formed is saturated.
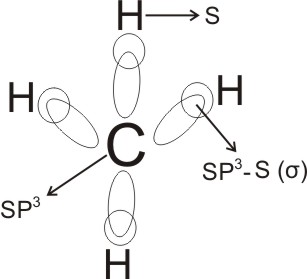
Alkanes are therefore tetrahedral in shape - the four covalent bonds are
directed to the four corners of a regular tetrahedron, the bond angle is about
109.5o.
Note:
- The structure of alkanes is not flat as is usually written (for quick
writing), but tetrahedral as shown above.
- When carbon atoms form single bonds, they use sp3 hybridization.

