|
Home
Alkynes: Ethyne, Properties, and Uses
Alkynes are hydrocarbons which form a homologous series with the general molecular formula CnH2n- 2. Where n is from 2 upward.
All members of the alkynes possess at least one triple bonds between two adjacent carbon atoms (- c
º
c - ) . The first and the simplest member of the group is n = 2
(i.e., C2H2 - ethyne).
The presence of the triple bond (i.e., 3 pairs or 6 electrons) is an indication of very high level of unsaturation, hence alkynes are more unsaturated than
alkenes.
Structure of Alkynes
In alkynes, carbon atoms which form the triple bond use sp hybridization. I.e., the one 2s orbital mixes with one of the three 2p orbitals to form two atomic orbitals of equal or equivalent energies.
The simplest hydrocarbon carbon can form with sp hybridization is one with two carbon atoms (e.g., ethyne). The hybridized orbitals are used in forming sigma bonds - one between each carbon, the other between each carbon and hydrogen atom.
The two unhybridized 2p-orbitals in both carbons overlap with one another in a plane above and below the single bond, to form altogether a triple bond (high unsaturability) between the two carbon atoms.
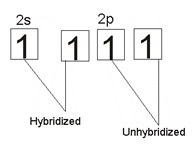
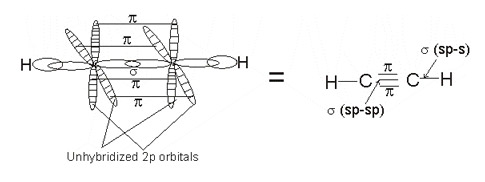
The simplest member, ethyne is used as a representative of the group - its structure and properties are general for all members.
The structure of ethyne is Linear, with bond angle 1800:
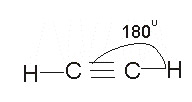
Note: when carbon atoms form triple bonds, they use sp hybridization.
Production of Ethyne from Action of Water on Carbides
Ethyne can be prepared in the laboratory by passing cold water on calcium dicarbide.
The reaction produces much heat.
CaC2(s) + 2H2O(l)
®
Ca(OH)2(aq) + C2H2(g)
Note:
*The use of acidified CuSO4 solution is to remove the chief impurity phosphine, PH3.
*Great heat which could break the containing flask is released from the reaction. The flask is therefore protected by filling its bottom with sand.
Properties of Ethyne
Physical properties:
1. Ethyne is a colorless gas with a sweet smell when pure.
2. It is slightly less dense than air (v.d. = 13).
3. It is almost insoluble in water.
4. It burns with a sooty flame due to high proportion of unburnt carbon.
5. It is strongly endothermic, hence, it is very unstable, and is likely to explode if stored
under compression alone. Therefore, for
industrial purposes, it is stored in steel cylinders in solution in propanone at about 12 atm pressure.
Chemical reactions:
The site of most chemical reactions in ethyne is the - C
º
C -, hence this is the functional group of the alkynes in general.
Due to the high degree of unsaturation, most of the reactions of ethyne are addition reactions.
A molecule of ethyne is completely saturated by accepting a maximum of four atoms of hydrogen or its equivalent.
| HC
º
CH + AB
|
® |
AHC = CHB + AB
|
® |
A2HC - CHB2 |
Note:
*The two A groups usually combine with the same carbon atom, similar to the two B groups. It is not common to produce compounds of the type ABHC - CHAB.
*The reaction is in two stages - stage 1 is the formation of the corresponding alkene derivatives, while the second stage is the formation of the completely saturated alkane derivatives.
(a). With hydrogen - in the presence of nickel catalyst at about 2000C, ethyne reacts with excess hydrogen to form a completely saturated hydrocarbon, ethane.
| H -C
º
C -H + H2
|
® |
H2C = CH2 + H2
|
® |
C2H6 |
Note:
The reaction will stop at the production of ethene (i.e., stage1) if limited quantity of hydrogen is used.
(b). With the halogens - ethyne combines rapidly with chlorine and bromine in the presence of a catalyst (metallic halide- FeCl3) to produce halogenated compounds.
Notice that a catalyst is used here, rather than the direct addition of halogens to alkenes. The use of the catalyst is to
polarize the halogen molecule and enhance its electrophilic character.
Note:
Chlorine will only react with ethyne to form the above products if it is diluted with an inert gas, but if pure, a violent explosion
occurs, resulting in the formation of carbon and hydrogen chloride - this is due to the strong affinity between hydrogen and chlorine.
C2H2(g) + Cl2(g)
®
2C(s) + 2HCl(g)
(c). With halogen acids or hydrogen halides
Ethyne reacts very readily with excess hydrogen iodide at room temperature to form 1,1-diiodoethane.
H-C
º
C-H(g) + HI(g)
®
IHC = CH2(i) + HI(g)
®
I2HC-CH3(I)
Note:
*For the second stage of reaction, the electronegative element is added to the carbon with lower number of
hydrogen.
*With HBr and HCl, a corresponding reaction is given, but slower.
The
order of reactivity of hydrogen halide with alkyne is HI > HBr > HCl.
This is because the H-I bond is the
easiest to break than HBr and H-Cl.
Hence HBr reacts faster than HCl.
(d). With water
Water is added onto ethyne in the presence of dilute H2SO4 and mercury(II) tetraoxosulphate(VI) as a catalyst at about 600C to produce ethanal (acetaldehyde).
C2H2(g) + H2O(l)
®
CH3CHO(l)
Note: The above reaction (d) is very important as it gives the industrial preparation of ethanal, which can then proceed to produce ethanoic acid.
(e). With potassium tetraoxomanganate(VII)
Ethyne, being unsaturated like the alkenes, decolorizes acidified solution of KMnO4, while the alkaline solution of KMnO4 is changed to green at room temperature.
The reaction is the oxidation of ethyne to ethanedioc acid (oxalic acid).
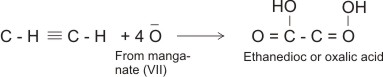
Note:
*This reaction distinguishes ethyne (i.e., alkynes) from all gaseous alkanes, e.g., methane and ethane, which do not react with KMnO4 solution.
*Also, the reaction with bromine vapor distinguishes ethyne from the alkanes, as ethyne decolorizes the reddish brown color of bromine vapor, but the alkanes do not - this is a more reliable test for an unsaturated compound than the use of KMnO4
solution (because any reducing agent which might not be unsaturated can also
decolorize KMnO4).
Substitution reaction in ethyne:
Ethyne can also undergo substitution reactions. This they do with sodium in liquid ammonia, and with certain salts of heavy metals to form metallic derivatives:
1. With sodium in liquid ammonia - hydrogen is displaced.
| 2CH
º
CH liq.NH3
|
Na® |
2HC
º
C- Na+ + H2(g)
|
|
|
Note:
This reaction shows that terminal alkynes show acidic
behavior.
2. With ammonical solution of copper(I) chloride at room temperature:
A reddish-brown precipitate of copper(I) dicarbide is formed.
C2H2(g) + 2CuCl(aq)
®
Cu2C2(s) + 2HCl(aq)
3. With ammonical solution of AgNO3 at room temperature:
A whitish-yellow precipitate of silver dicarbide is formed.
C2H2(g) + 2AgNO3(aq)
®
Ag2C2(s) + 2HNO3(aq)
Note:
*Both dicarbides, if dried and heated are explosive.
*Both give off ethyne when warmed with
dilute acid.
Ag2C2(s) + H2SO4(aq)
®
Ag2SO4(s) + C2H2(g)
*Ethyne is distinguished from ethene by the above reactions -
i.e., the formation of metallic derivatives (ethene does not form any
metallic derivative).
*All alkynes which have a tripple bond at the end of their carbon chain (i.e. terminal alkynes) would undergo similar substitution reactions, hence, they are
distinguishable from the alkenes (which do not).
2CH3CH2-CºCH + 2CuCl
®
2CH3CH2-CºCCu + 2HCl
Combustion of Ethyne
Ethyne burns readily in oxygen, releasing enormous amount of heat, which can be used in cutting and welding metals.
This flame is called oxy acetylene flame, used by welders.
The products of the reaction are carbon(IV) oxide and steam.
2C2H2(g) + 5O2(g)
® 4CO2(g) + 2H2O(g)
Polymerization
of Ethyne
By passing ethyne through a heated metal tube at 400oC and 1,013 x105 N M-2, ethyne is polymerized to benzene.
| 3CH
º
CH
|
400oC
® |
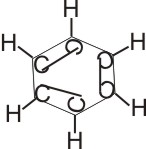 Benzene Benzene
|
|
|
Uses of Ethyne
(1). As a source of polyvinyl chloride (PVC) plastic:
Vinyl chloride (H2C=CHCl, chloroethene) is formed from the reaction between ethyne and hydrogen chloride .The vinyl chloride is polymerized to give a plastic product which is used in electrical insulation, water-proofing and in the manufacture of pipes.
(2). For the production of hot flame:
Oxyethyne
(or oxy-acetylene) flame is very hot and is used for cutting and welding metals. It is produced by mixing ethyne with oxygen.
(3). As a source of important organic compounds:
1,1,2- trichloroethane and 1,1,2,2, - tetrachloroethane are used as solvent for grease, fats, and oil - they are produced from ethyne.
Also, ethanal and ethanoic acid and its derivatives are products of ethyne.
(4). As lamps:
Certain lamps, e.g. hunter’s and miner’s lamp are fuelled by ethyne.
(5). In the production of polypropenonitrile:
Propenonitrile (CH2=CHCN) polymerizes in the presence of a peroxide initiator to produce important synthetic
fiber known as polypropenonitrile.
... - CH2-CHCN-CH2-CHCN-...
(6). In the production of neoprene (polymer of
2-chlorobuta-1,3-diene):
Neoprene is an artificial rubber which does not burn or attacked by oils.
|

