|
Home
Alkenes
The alkenes are another group of hydrocarbons that forms a homologous series where all members
conform to the general molecular formula CnH2n.
where n is equal to or greater than 2.
Formula of Alkenes
The formula of any member of the alkene hydrocarbon group can
be determined by using the formula CnH2n. Therefore, the first
member, with carbon atom number 2 is ethene, which gives the formula C2H4 ; and the second
is propene with the formula C3H6.
Notice that alkenes are two hydrogen atoms less than their corresponding
alkanes. Alkenes are therefore unsaturated - they do not contain the
maximum number of hydrogen needed to saturate them.
Structure of Alkenes
In alkenes, carbon atoms which form the double bonds use
sp 2
hybridization. I.e., the one 2s orbital is mixed with two of the three 2p orbitals to form three atomic orbitals of equal energies.
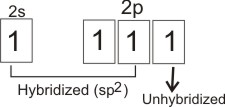
One of the 2p orbitals is unhybridized.
The simplest
hydrocarbon carbon can form with this type of hybridization is one having two
carbon atoms with the same type of hybridization - sp2.
The three hybridized orbitals are used to form real bonds known as sigma (s) bonds - one with the adjacent carbon atom (sp2-
sp2), and
the other two separately with the s orbital of two hydrogen atoms (in ethene).
The unhybridized 2p orbitals of the two atoms overlap with each other to
form a kind of bond called pi (p) bond - the presence of this bond (electrons in a plane above and below the
single bond) gives rise to the unsaturability in alkenes.
orbital diagram in ethene:
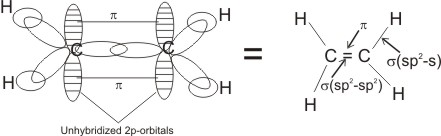
Ethene is the simplest and the most important member of the
alkenes. It is used in illustrating the structure, methods of preparation and
chemical reactions of members of the group. The structure of ethene is again
given below.
Ethene is trigonal planar in shape, with bond angle 120O.
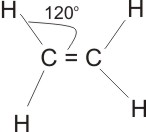
Note: * All alkenes must possess at least one
double bond between two adjacent carbon atoms ( - C = C -), this is the functional group of
the alkenes.
* Sigma bonds (s)
- these are bonds between hybridized orbitals or between hybridized orbitals and s orbital.
* Pi-bond (p)
- these are bonds between unhybridized orbitals.
* When carbon atoms form double bonds, they use sp2
hybridization.
Laboratory Preparation of Alkenes (E.g. Ethene)
1. Ethene can be prepared in the laboratory by a process involving the
dehydration of ethanol using hot conc. H 2SO4.
The mixture of the two is heated to 1700C
and the dehydration is effected.
C2H5OH(aq) +
conc.H2SO4
- H2O ®
C2H4(g)
Note: * The dehydration process goes through two
stages: the initial formation of ethyl hydrogen tetraoxosulphate(VI)
C2H5OH(aq) + H2SO4(aq)
®
C2H5HSO4(aq) + H2O(l); and the decomposition of the ethyl hydrogen tetraoxosulphate(VI)
on heating, C2H5HSO4(aq)
® C2H4(g)
+ H2SO4(aq)
* Sodium hydroxide, NaOH solution is used in the process to absorb possible
impurities, such as SO2 (produced from partial decomposition of H2SO4)
2. By the elimination of halogen and hydrogen (dehydrohalogenation)
- elimination of hydrogen halides from haloalkanes is possible by treating the
haloalkane with an alcoholic solution of potassium hydroxide. Example, from primary alkylhalide - only terminal alkenes are formed.
From secondary alkyl halides:
Mechanism of reaction:
and
Both products are formed together, however, the internal
alkenes are more stable and are therefore formed more.
3. By elimination of halogen (dehaloge- nation) - when
an alkylhalide, with the halides on two adjacent carbon atoms is heated with a
metal, the two halides are lost, resulting in the formation of alkenes. Example:
Industrial Preparation of Ethene
Ethene is produced in commercial quantity from the cracking
of the gas oil fraction obtained from fractional distillation of petroleum.
C17H36(l)
®
C8H18(g) + 3C2H4(g) + C3H6(g)
Note: other members of the alkene group can be
prepared by similar procedure as above, by using the corresponding alkane
derivatives.
Properties of Ethene
Physical properties:
1. It is a gas at room temperature, colorless and with a
faint sweetish smell.
2. Almost insoluble in water.
3. Slightly less dense than air (v.d. = 14).
4. Neutral to litmus.
Chemical reactions of ethene:
Combustion - if ignited by an electric spark or light,
ethene burns or explodes in air with a smoky flame. The flame is due to unburnt
carbon (because of its high proportion -about 85%). The product of combustion is
steam and carbon(IV) oxide.
C2H4(g) + 3O2(g)
® 2CO2(g) +
2H2O(g)
Addition reactions:
The characteristic reaction of ethene is addition
reactions. Ethene is unsaturated. The presence of the double bond
(i.e.
p
-
bond electrons in a plane above and below the single bond) provides readily
available electrons for two more hydrogen atoms or its equivalent to add on to
its molecule, thereby saturating it.
Some of the common addition reactions of ethene are:
(a). With hydrogen - in the presence of finely divided
nickel, paladium or platinum as catalyst, ethene combines with hydrogen to form
ethane.
|
H2C = CH2(g) + H2(g)
® CH3CH3
or C2H6(g)
ethene ethane |
|
|
|
|
Note: * This reaction is known as hydrogena-tion, and it is the important reaction which leads to the hardening of
vegetable oils into margarine. Oils consist of a long
carbon chain (C15
- C17)
with one terminal - C=C - bond.
The double bond gets attacked and saturated by
the hydrogen, resulting in the hardening of the oil, i.e., the vegetable oil is
converted into a fat.
* Fats are more difficult to burn by the body because
they are saturated hydrocarbons (saturated hydrocarbons are inert or unreactive).
- CH = CH - + H2
® -CH2
- CH2 -
(b). With chlorine or bromine vapor - at room
temperature, an oily liquid is formed readily between chlorine and ethene.
|
H2C
=CH2(g) +
Cl2(g)
® CH2ClCH2Cl
1,2-dichloroethane |
|
|
|
|
With bromine, similar reaction occurs, resulting in the
decolorization of the reddish - brown color of bromine vapor.
Note: the decolorization of bromine vapor by ethene
is used to distinguish ethene or any other unsaturated hydrocarbons from any
gaseous alkane (example, methane or ethane, which do not decolorize bromine vapor).
|
H2C
= CH2(g) + Br2(g)
®
CH2BrCH2Br
1,2-dibromoethane
|
|
|
|
|
(C). With hydrogen halides - the reaction between
ethene and hydrogen iodide vapor occurs readily to form iodoethane.
CH2
= CH2(g) + HI(g)
® CH3CH2I
Similar reactions occur with HBr and HCl but less readily.
(d). With chlorine and bromine water. Chlorine water
contains chloric(I) acid HOCl. It combines with ethene to produce 2-chloroethanol.
In the same way bromine water, contaning bromic(I) acid, HOBr
will form 2- bromoethanol with ethene.
(e). With conc. H 2SO4
- ethene is readily absorbs at room temperature by the acid to form ethyl
hydrogen tetraoxosulphate(VI). When boiled in water, the ethyl hydrogen
tetraoxosulphate(VI) yields ethanol and the tetraoxosulphate(VI) acid.
C2H4(g)
+ H2SO4(aq)
® C2H5HSO4(aq)
C2H5HSO4(aq)
+ H2O(l)
® C2H5OH(aq) + H2SO4(aq)
(f). With potassuim tetraoxomanganate(VII) - ethene
decolorizes the purple color of acidified solution of KMnO4
(being reduced to the manganate(II) salt).
With alkaline solution of the KMnO4, ethene changes its color from purple to green (being reduced to manganate(VI)
salt).
Ethene in the course of the reaction is oxidized to ethane
-1,2- diol (glycol) by water and oxygen (from the KMnO4)
CH2 = CH2(g) + H2O + (O)
® CH2OHCH2OH(aq)
Note:
* By the above reaction, (f), ethene can be
distinguished from all gaseous alkanes, such as methane or ethane which are
saturated and would not be oxidized.
* The product, ethane-1,2- diol (glycol) is used in
anti-freeze solution for motor car radiators and for the manufacture of an
important polyester called terylene.
(g). With air or oxygen: ethene reacts with oxygen of
the air when passed over a silver catalyst at about 250 0C.
The product of the reaction is epoxyethane (ethylene oxide).
|
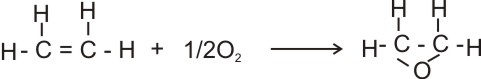
epoxyethane |
|
|
|
|
Note:
* Epoxyethane is useful in the production of certain liquid detergents and ethane -1,2- diol.
* Generally, notice that the end product of the
chemical reactions of ethene are saturated products of the alkane derivatives.
* All other alkenes show the above chemical reactions,
but to different degrees.
Naming Alkenes
Alkenes possessing 4 carbon atoms and above are usually named
by their I.U.P.A.C. system of naming, while the simple members are named by
their common names. The I. U. P. A. C. system of naming alkenes is similar to
that of the alkanes.
1. The parent name or structure is the longest continuous
chain of carbon atoms containing the olefinic bond (=).
2. The parent name is the replacement of "-ane" ending of the
corresponding alkane by "-ene"
Example,
|
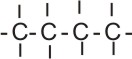
butane |
|
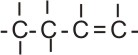
butene |
|
|
3. The numbering of the carbon atoms in the longest
continuous chain is done such that the carbon atoms bearing the olefinic bond
(=) are assigned the lowest positions, example:
|
C -
C - C = C
4 3
2 1 |
and not C - C- C = C
1 2 3 4 |
|
|
|
4. The position of the olefinic bond is indicated in the name
by choosing the lower number of the two carbon atoms bearing it.
Example, .
(name:1- butene or but-1-ene and not 1,2- butene or 2- butene)
5. If there is (are) any substituent groups attached to the
longest continuous chain, indicate them by the order of numbering as in the
alkanes.
However, notice that the substituents do not determine the numbering
order, rather, it is the position of the olefinic bond which does.
Example,
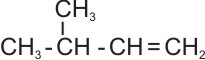
The numbering is
|
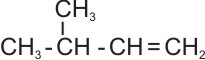 4 3 2 1 4 3 2 1 |
|
and not |
|
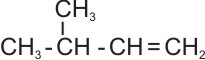
1
2 3
4 |
Therefore, the name is 3-methyl-1-butene or 3-methylbut-1-ene.
6. If there are more than one double bonds in the longest
chain, the prefixes di-ene (for 2), tri-ene (for 3) and tetra-ene(for four) ends
the naming. However, the positions of the double bonds have to be indicated.
Example,
|
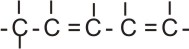 |
pent-1,3-diene |
|
|
|
Related:
Isomerism in Alkenes
Polymerization Reactions of Alkenes
|

