|
Home
Corrosion as an Electrolytic Process
Corrosion in metals have been found to be electrochemical process – simple half-cell reactions are involved. Example,
the corrosion of iron (also called rusting).
Rusting in iron is promoted by the following factors:
(a). The presence of water and air.
(b). The presence of H+ ions, which speeds up the
process.
(c). The presence of less reactive metals such as tin and copper.
(d). The presence of strain points in the metal.
(e). The presence of certain salts solutions, which are believed to be catalytic in their reactions.
Rusting process can be
explained thus:
1. The presence of less reactive metals as impurities in the iron set up a potential difference at a strain point between iron atoms, and in the presence of water, the iron readily loses electrons in the aqueous solution, which acts as electrolyte.
I.e. Fe(s) → Fe2+(aq) + 2e-(aq)
Hence, the iron becomes a
kind of an anode while the cathode is the metal impurities.
The iron(II) ion in the solution is readily oxidized by oxygen to iron(III) oxide, which becomes hydrated.
The overall reaction may be represented as:
4Fe + 3O2 + 2xH2 → 2Fe2O3 . xH2O
Note: Fe2O3 . xH2O is the rust.
Corrosion affects all reactive metals. i.e., metals that can easily oxidize.
M(s) → Mx+ + xe- - this process enables corrosion to affect them.
Examples of such metals with relative high oxidation potential are Al, Zn and Fe.
The rust layer on the iron is soft and porous, allowing water and air to continue the rusting process, unlike the corrosion layer of oxides of metals like Al and Zn.
Pure ion will not rust, because the possibility of local potential difference promoted by impurities will not arise.
Prevention of Corrosion
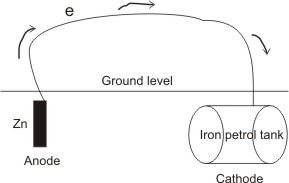
Corrosion will be prevented if at least one of the processes involved in causing it is disallowed from occurring. Thus, all methods used to prevent corrosion are based on this principle. The most effective method of preventing corrosion in iron is the cathodic protection.
By this method, a more electropositive (or more reactive) metal (e.g. Zn or Mg) is made the anode and the iron, the cathode. A conducting wire is connected between them. Thus, the iron is prevented from going into solution as Fe2+, i.e. the reaction:
Fe(s) → Fe2+(aq) + 2e-(aq) is prevented from taking place.
I.e. Anodic reaction: Zn(s) → Zn2+(aq) + 2e-(aq)
Cathodic reaction: Fe2+(aq) + 2e-(aq)
→ Fe(s)
This method is applied in the protection of buried iron petrol tank reservoir from rusting.
the iron. Also, Zn2+ reacts with oxygen to form a film of zinc oxide, which protects the surface from further corrosion.
Other methods of preventing corrosion are:
(1). Galvanizing with zinc:– Zinc is used to
galvanize (i.e. coat) iron in steel to prevent rusting of pipes in many plumbing systems. In the presence of moisture and when
galvanized iron is scratched, the Zn being higher in the
electrochemical series losses electrons and goes into solution as Zn2+ ions – it therefore corrodes instead of
iron
(2). Tinning: - Tin is used to protect iron from rusting, as in tin cans. This is possible because tin is very resistance (i.e. unreactive) to chemical attack.
However, this is only sustained if the can is not scratched. If it is scratched, a very high potential difference is set up between the tin and iron, with the tin pulling electrons from iron, thereby causing the reaction Fe→ Fe2+ +2e- to occur (i.e. iron begins to rust and at a faster pace than usual).
(3). Electroplating: - This is the electrical precipitation of one metal onto the surface of another to give improved appearance or greater resistance to corrosion. The metal to be electroplated is made the cathode and the anode is the metal (pure), which is required.
A direct current is passed through the solution (electrolyte, which is a solution of the salt of the required metal). Example: in electroplating a metal with silver, the cathode is the metal (iron) to be electroplated, the anode is the pure silver and the electrolyte is either sodium or potassium dicyanoargentate(I) solution, KAg(CN)2.
By passing a direct current into the solution, the following reactions occur at the electrodes:
Cathodic reaction:
Ag+(aq) + e-(aq) → Ag(s)
Silver deposited on the cathode (iron)
Anodic reaction: Ag(s) → Ag+(aq) + e-(aq) - Silver dissolved
Note: The process is the transfer of pure Ag from the anode to the cathode.
(4). Greasing, painting or spraying the iron bar – the essence is to prevent water and oxygen from getting to it.
(5). By surrounding the metal (iron) with fumes from a material that discourages rust, such as camphor (C10H16O) or mothballs.
Some mothballs are naphthalene (C10H8) while others are Para dichlorobenzene.
(6). By storing iron is a tight wooden box – wood acts as a fairly good barrier to humidity and absorbs moisture.
(7). By preventing the gathering of dust on iron - dust absorbs moisture.
|

