|
Home
Petroleum and Petrol
Petroleum is a mixture of natural gas and crude oil. It is believed to be formed by the decomposition of
deposits of dead marine organic matter by the action of bacteria, heat and pressure millions of years ago.
Composition
Petroleum is composed mainly of a mixture of hydrocarbons (about 90%) together with a small
proportion of compounds containing sulphur, oxygen, and nitrogen (about 10%).
Fractional Distillation of Petroleum Components
The different components of petroleum are separated by fractional distillation, i.e. the difference in the boiling points of the components is used as a basis for separating them.
The table below gives the major fractions obtained by the fractional distillation of crude oil, their
boiling points and uses.
| Fraction |
Number of Carbon Atoms
per Molecule |
Boiling Point Range
OC |
Uses |
| Natural gas |
1- 4 |
Below 20 |
Fuel for heating and lighting, for
the manufacture of certain compounds, such as ethyne, hydrogen,
carbondisulphide, and tetrachloromethane. |
| Petroleum ethers and
ligroin |
5 - 7 |
20 - 120 |
As organic solvent |
| Petrol (gasoline) |
5 - 12 |
40 - 205 |
As motor and aeroplane fuel, for
heating and lighting, and as solvent for grease and paints. |
| Kerosene (paraffin
oils) |
12 - 18 |
175 - 325 |
For heating and lighting; fuel for
jet engine, and for driving tractors. Also as solvent for grease and
paints. |
| Gas oil and diesel oil |
12 - 25 |
275 - 400 |
As fuel for diesel engine, for
heating, and as raw materials in the cracking process. |
| Lubricating oils,
waxes, grease, and vaseline |
Above 20 |
Above 400 |
Lubrication, medicine, manufacture
of candle, polish, ointments, creams, and water proofing materials.
|
| Bitumen, asphalt, and
other residue |
Above 40 |
Above 400 |
For surfacing roads and air
fields; other residues may be used as fuel, as protective pipe coating,
and as paint. |
Cracking of Petroleum
Cracking of petroleum is
the breakdown of hydrocarbon fractions (alkanes) with large number of carbon
atoms into smaller molecules, or the conversion of straight chain hydrocarbons
to branched-chain hydrocarbons.
There are two methods of cracking - thermal
(carried out by heating to high temperatures of about 700oC
and pressures of about 30 atm) and catalytic (the use of aluminium oxide and
silicon(IV) oxides as catalyst).
Reason for Cracking
1. To obtain large quantity of petrol - i.e., to
produce more of small molecular hydrocarbons, which are in the C5
- C12
range. Petrol is high in demand, but is produced from the fractional
distillation of crude oil in small quantity.
2. To produce petrol of higher quality - i.e., to
convert straight- chain petrol to branched-chain petrol. The branched-chain
petrol burn more smoothly, hence they are more efficient in an internal
combustion engine than the straight-chain petrol which is produced more from the
fractional distillation of crude oil.
Examples of reactions that occur during cracking process:
1. Conversion of large molecular alkane to high grade petrol
containing branched-chains.
C
C16H34
® C8H18
+ C8H16
Branched
octane
Octene
|
|
|
|
|
2. Conversion of a straight chain alkane to a branched-chain.
|
C7H16
®
|
CH3CH(CH3)CH(CH3)CH2CH3
2,3-dimethyl Pentane |
|
|
|
3. Conversion of a straight chain alkane to a
branched chain alkane and an alkene.
CC8H18
® CH3CH(CH3)CH2CH2CH3
2-methyl pentane |
+ C2H4
Ethene |
|
|
|
4. Conversion of a straight chain alkane to a cycloalkane and
hydrogen gas
|
C4H10
®
|
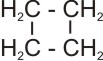
Cyclobutane |
+ H2(g) |
|
|
Note :
cracking process yields also several gases as
by-products, e.g., hydrogen, ethene, benzene, ethane, butene, propene and
propane, which can be used in reforming alkanes in the petrol range, and as raw
materials in the petrochemical industry for the manufacture of several useful
chemicals and products.
Example, hydrogen is used for the manufacture of ammonia;
ethane for ethanol; benzene and propene for the manufacture of drugs,
detergents, plastics, synthetic rubber, fertilizers, weed killers and synthetic
fibres.
Reformation of Petrol
Hydrocarbons in the petrol range, as well as special fuels
(e.g. high octane aviation spirit), can be reformed from gaseous hydrocarbons.
The processes which can be used in carrying this out are:
(1) polymerization (2) alkylation (3) isomerization and (4)
hydrogenation. These are summarized below:
1. Polymerization and hydrogenation
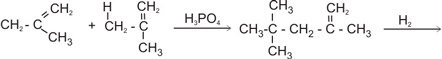
Isobutene Isobutene
Iso-octene |
Iso-octane |
|
|
|
2. alkylation

isobutane
isobutene iso-octane |
|
|
|
|
|
(methyl propane)
|
(methyl propene) |
(2,2,4-trimethylpentane) |
|
|
3. isomerization
Quality of Petrol and Meaning of Octane Number
The quality of petrol is a measure of how it burns in an
internal combustion engine. A poor quality petrol burns rapidly and unevenly and
generates an explosion which disturbs the up and down movement of the piston,
resulting in a strange sound normally referred to as ‘Knocking’ - this petrol
contains large proportion of straight chain alkanes.
A good quality petrol on the other hand burns smoothly and
evenly - this contains a large proportion of branched alkanes.
Note::
A given petrol consists of both branched and
straight chain alkanes.
The octane number of a petrol is an arbitrary number which
denotes the proportion of the branched-chain to the straight chain alkanes in
the petrol. A scale which ranges from zero (for n-hepane - straight chain
alkane) to 100 (for 2,2,4 - trimethyl pentane - branched chain alkane) has been devised.
Hence, a petrol with an octane
number of 95 consists of 95 parts of the branched alkane (2,2,4 - trimethyl
pentane) and just 5 parts of the straight-chain alkane (n-heptane) - this is a very high quality petrol and
is regarded as a ‘five star’ or ‘super’ petrol. The octane rating of the regular
petrol is between 85 and 92.
Uses of Petrol Additives
High octane rated petrol is very expensive and might not be readily
affordable, especially in the third world countries. Certain chemicals have been
developed to be used as additives. These chemicals function to increase the
octane rating of low grade petrol by slowing the combustion
rate of the petrol - they are therefore called anti-knock.
Examples of
petrol additives are tetraethyl lead(IV) (TEL), Pb(C(C 2H5)4;
methanol and ethanol.
Note : Tetraethyl lead(IV) is no longer in use, especially in the
developed countries due to its environmental pollution effect - it introduces
lead into the environment, thereby causing severe environmental and health
hazards.
Related:
Hydrocarbon and Alkanes
Methane
Isomerism
in Alkanes
IUPAC Nomenclature of Alkanes
|

