|
Home
Organic Acids | Carboxylic Acids
All organic acids contain at least one carboxyl group, which is why they are also
called carboxylic acids.
The Carboxyl Group
The carboxyl group is made up of the carbonyl group (C =O) and the hydroxyl group (-OH).
It is the functional group that determines the chemical properties of the
carboxylic acids.
Hence, the -COOH group is the functional group and they are called carboxylic acids.
Classification and Structure of Organic Acids (Carboxylic Acids)
Organic acids can be classified into two groups: aliphatic and aromatic carboxylic acids. Aliphatic carboxylic acids are called alkanoic acids.
Structurally, the - COOH group is attached to alkyl groups.
There are different kinds of alkanoic
acids- some contain one carboxyl group per molecule- these are called monocarboxylic acids.
Others contain more than one carboxyl group.
E.g.
two - dicarboxylic acids, and three- tricaboxylic acid.
E.g.
CH3COOH
Ethanoic acid
(monocarboxylic acid) |

Ethanedioc
acid or oxalic acid (dicarboxylic acid) |
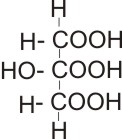
2-hydroxypropan-1,2,3-tricarboxylic acid
(tricarboxylic acid) |
|
|
Aromatic Carboxylic Acids
These compounds have the carboxyl group - COOH attached to aryl or ring structures. E.g.
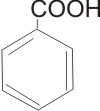
Benzoic acid |
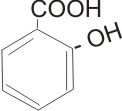
2- hydroxybenzoic acid |
|
|
|
Carboxylic Acids Formula
Of the above different classes, the monoaliphatic carboxylic acids are the most common. They form a homologous series with general molecular formula
CnH2n + 1 COOH or CnH2nO2
The first five members are given below:
n = 1
HCOOH Methanoic acid
n = 2 CH3COOH
Ethanoic acid
n = 3 CH3CH2COOH Propanoic acid
n = 4 CH3CH2CH2COOH Butanoic acid
n = 5 CH3(CH2)3COOH Pentanoic acid |
|
|
|
|
Nomenclature (Naming) of Carboxylic Acids
The I.U.P.A.C. system of naming a carboxylic acid is by selecting the longest
continuous carbon chain containing the carboxyl group.
The name of the corresponding alkane is replaced with -oic acid. The position of substituents
are indicated by numbering the longest chain from the carbon bearing the
carboxyl group.
E.g.
HCOOH
Methanoic acid |
CH3COOH
Ethanoic acid |
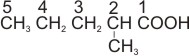
2- methylpentanoic acid |
|
|
Methods of Preparation of Carboxylic Acids
Carboxylic acids can be prepared both in the laboratory and in commercial
quantity following certain methods. To show how this type of compounds can be
made, we will use the ethanoic acid as an example.
Laboratory preparation of Ethanoic Acid
As we discussed earlier in the study of
alkanols, ethanoic acid is the final organic oxidation product of ethanol.
Thus, the process which will lead to the final oxidation of ethanol is applied (the oxidation occurs in two stages):
ethanol
®
ethanal
®
ethanoic acid
In the laboratory, the process can be carried out by heating ethanol with potassium heptaoxodichromate(VI) in dilute tetraoxosulphate(VI) acid.
CH3CH2OH(aq)
®
CH3 CHO(aq) + 2H+(aq) + 2e-(aq)
CH3CHO(g) + H2O(l)
®
CH3COOH(aq) + 2H+(aq) + 2e-(aq)
Another method, which is easier than the above is the distillation of anhydrous sodium ethanoate with concentrated H2SO4.
CH3COONa(s) + H2SO4(aq)
®
NaHSO4(aq) + CH3COOH(g)
Note:
* Hydrochloric acid is not used
because it is volatile and would therefore distill with the ethanoic acid.
* Hot ethanoic acid vapor attacks cork or rubber stoppers - the
apparatus must all be glass.
Industrial Manufacture of Ethanoic Acid
(1). From Butane - butane is oxidized directly to ethanoic acid by mixing it with air and passing it into ethanoic acid kept at 160OC in the presence of manganese(II) ethanoate as catalyst at 20 atm pressure.
(2). From Ethyne - ethyne is passed into dilute H2SO4 in the presence of mercury(I) sulphate(VI) as catalyst at about 95OC to produce ethanal vapor which is then mixed with air and passed over heated manganese(II) ethanoate as catalyst at 60OC.
C2H2(g)
®
CH3 CHO(g)
CH3CHO(g) + 1/2 O2(g)
®
CH3 COOH(g)
(3). From Ethanol - ethanoic acid is manufactured in the form of vinegar (contains 4-5% of ethanoic acid). The process involves the running of dilute aqueous solution of ethanol (such as wine of pure quality) over coke in a well aerated vat.
A bacteria - mycoderma aceti, which acts as catalyst is introduced into the coke. The liquid mixture is then passed into the vat several times to ensure complete oxidation of the ethanol to ethanoic acid.
CH3CH2OH(aq) + O2(g)
®
CH3COOH(aq) + H2O(l)
Carboxylic Acid Properties
To demonstrate the common properties shown by the carboxylic
acids, we will use ethanoic acid, which is a great example of the family of
compounds with the carboxyl functional group.
Properties of Ethanoic Acid
Physical properties
1. Ethanoic acid is usually a colorless liquid with a characteristic sharp and pungent smell.
2. It is very soluble in all proportion in water - it shows greatest solubility when compared with alkanes and alkanols of similar molecular weight.
Note: The reason for their relative high solubility in water is the presence
of high degree of hydrogen bonding, which are formed from C=O and -OH (two
points on a molecule of the carboxyl group, - COOH) with water molecules.
3. The pure anhydrous ethanoic acid has melting point of 17OC and freezes readily into ice-like crystals during winter in cold countries - hence it is called glacial ethanoic or glacial acetic acid.
4. It has a relative high boiling point of 118OC compared with alkanes and alkanols of similar molecular weight. This is due to high degree of inter-molecular attraction (i.e. hydrogen bonding) between opposite ends of carbon-oxygen and
carbon-hydroxy dipoles.
I.e., two hydrogen bonds are formed by each molecule with another, while in alkanols, one hydrogen bond is formed per molecule, and in alkanes no hydrogen bond is formed.
5. As an acid, it turns blue litmus red.
Chemical properties (reactions of ethanoic acid)
1. Acidic behavior - ethanoic acid slightly ionizes in dilute solution to furnish hydrogen ions H+, hence it is a weak acid.
|
CH3COOH(aq)
|
 |
CH3COO- (aq)+ H+(aq) |
|
|
The usual acidic behavior is as a result of the presence of H+ ion in its solution. I.e.,
(a). It liberates CO2 from carbonates, and hydrogen carbonates.
2CH3COOH(aq) + CaCO3(s)
®
(CH3COO)2Ca(aq) + H2O(l) + CO2(g)
(b). It is attacked by strongly electropositive metals, e.g. magnesium and calcium to release hydrogen.
2CH3COOH(aq) + Mg(s)
®
(CH3COO)2Mg(aq) + H2(g)
(c). It turns blue litmus red.
(d). It neutralizes alkalis or bases to form salts (ethanoates) and water only.
CH3COOH(aq) + NaOH(aq)
®
CH3COONa(aq) + H2O(l)
Note:
* The presence of the carboxyl functional group - COOH is responsible for the exhibition of the above acidic properties. Hence, all substances with the
-COOH group, including the dicarboxylic and tricarboxylic acids show the above properties. The di and tri carboxylic acids behave as dibasic and tribasic acids respectively.
* Ethanoic acid is not easily decomposed by heat, it is not affected by
ordinary oxidizing, reducing and dehydrating agents - it is fairly stable.
2. Ester formation: Ethanoic acid reacts with an alkanol, e.g. methanol or ethanol in the presence of a little conc. H2SO4 or dry Hcl as catalyst to produce an ester. E.g.
CH3COOH(l) + C2H5OH(l)
|
 |
CH3COOC2H5(l)
ethylethanoate
(ester) |
+ H2O(l)
|
|
3. Formation of acyl chloride: When anhydrous ethanoic acid is brought in contact with phosporus(V) chloride in the cold, a rapid reaction occurs with the production of ethanoyl chloride (acetyl chloride) and a steaming fume of Hcl.
CH3COOH(l) + PCl5(l)
|
® |
CH3COCl(l)
ethanoyl chloride |
+ HCl(g) + POCl3(l)
|
|
Note:
Acyl
chloride
|
|
|
|
|
* PCl3 reacts similarly but less rapidly.
* The reaction shows the presence of the - OH group in the acid which is attacked by PCl5.
* The alkyl group - CH3 is unaffected.
4. With chlorine - in the presence of light as catalyst, if chlorine is passed into boiling ethanoic acid, monochloroethanoic acid will be formed.
Cl2(g) + CH3COOH(l)
®
CH2ClCOOH(l) + HCl(g)
Further passage of chlorine will form the dichloro and trichloro ethanoic acid respectively i.e.,
Cl2(g) + CH2ClCOOH(l)
®
CHCl2COOH(l) + HCl(g)
Cl2(g) + CHCl2COOH(l)
®
CCl3COOH(l) + HCl(g)
Note:
* The chlorine attacks only the alkyl (CH3) group.
* If monochloroethanoic acid is made to react with aqueous ammonia, the chlorine will be displaced by the amino (NH2) group to form glycine, H2NCH2COOH. Glycine is the simplest of the amino acids, which are the basic unit of proteins.
5. Decarboxylation reaction: Anhydrous ethanoic acid will produce methane and CO2 when heated with soda-lime.
CH3COOH(aq)
® CH4(g) + CO2(g)
Uses of Ethanoic Acid
(1). As a solvent.
(2). As vinegar - for flavoring and preserving foods in the food industry.
(3). For the production of many useful industrial chemicals. E.g. ethanoic anhydride - used in asprin and in the production of cellulose ethanoate; various dyes; propanone and ethenyl ethanoate - used in vinyl resins.
(4). For coagulation of rubber latex.
|

