|
Home
IUPAC Nomenclature of Alkanes
There is a general system for naming organic compounds instituted by the
International Union of Pure and Applied Chemistry, I.U.P.A.C.
Here are the rules to follow to
name hydrocarbons, using alkanes as examples:
1. Select the longest straight chain of carbon atoms in the molecular structure
- this gives the parent name of the compound.
2. Recognize any substituent on the longest chain. There are usually the
following alkyl substituent:
CH3 - methyl; CH3CH2 - ethyl; and CH3CH3CH2 - propyl.
3. Number the carbon atoms on the straight chain, such that those bearing the
substituent would have the lowest number.
4. If there are two or more same substituent, use the prefixes: di- for two;
tri-for three and tetra- for four, before the name of the substituent.
5. If there are two substituent on the same carbon, then the position of that
carbon is indicated twice before the names of the substituent.
6. Write the names of the compound as one word, separate numbers from
substituent groups and prefixes with a hyphen(-) and numbers from each other
with a comma (,).
The position (s) of carbon (s) bearing the substituent is (are)
written first, followed by prefixes, then the name (s) of substituent in
alphabetical order, then finally the parent name.
Example: name the following:
1. 
Solution: The longest straight chain is the one with 6 carbon atoms. Hence, the
parent name is hexane.
The substituent are two methyl groups (- CH3). The
correct numbering of the longest straight chain is
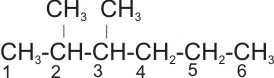
rather than
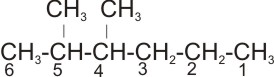
There are two same substituent groups, - the prefix- di is used. Therefore, the
name of the
compound is 2,3-dimethyl hexane.
2. 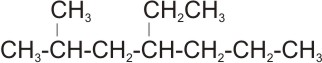
Solution: the longest straight chain is the one with seven carbon atoms- the
parent name is heptane. The substituent are: one methyl and one ethyl groups.
The right numbering is:
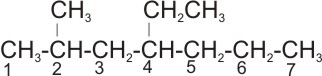
Positions 2 and 4
rather than
.
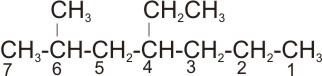
Positions 4 and 6
Therefore, the name of the compound is
4-ethyl-2- methyl heptane.
Note: ethyl is expressed first before methyl - expression of the substituent
groups is based on alphabetical order of their names.
3. 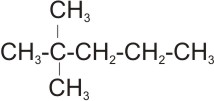
Solution: The parent name is pentane. The substituent are two methyl groups
attached to the same carbon (this carbon position will be indicated twice). The
correct numbering is from left to right, giving the carbon bearing the
substituent number 2. Therefore, the name of the compound is 2,2 - dimethyl
pentane.
4. 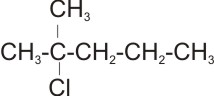
Name: 2-chloro -2 - methyl pentane
5. 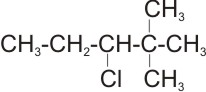
Name: 3-chloro-2,2-dimethyl pentane
Note: the prefix -di does not determine the order of expressing the substituent,
rather, it is the alphabetical order of their names.
Related:
Hydrocarbon and Alkanes
Methane
Isomerism
in Alkanes
Petroleum and
Petrol
|

