|
Home
Benzene, Properties, and Uses
Benzene, C6H6, was discovered by Michael Faraday in 1825. It is a hydrocarbon obtained from the distillation of coal tar. It is a member of a large family of organic compounds called aromatic compounds.
The term aromatic was initially used because these compounds possess certain characteristic odor (or aroma), but now, it is used to describe compounds which possess certain common features.
Some of these features are:
1. They are cyclic (i.e., ring) compounds.
2. Their molecules are planar.
3. They are unsaturated, and contain delocalized
electron clouds above and below the ring.
4. They obey Huckel’s rule - i.e., there are (4n+2) electrons involved in the delocalization, where n is the number of rings available.
Therefore, for
benzene (n =1) - there are 6 delocalized electrons in the ring.
5. They exhibit resonance.
Structure of Benzene
Benzene has a molecular formula of C6H6 which indicates it to be unsaturated.
In 1865, a
German chemist, August Kekulé, proposed that the structure of benzene is a ring called cyclohexatriene.
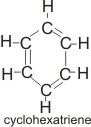
Although this was a great step forward, it could not explain the stability of the ring. However, modern methods for investigating chemical structures, such as X-ray
determination, have revealed that the benzene molecule is flat and that the six carbon-carbon bonds are of equal
length, 1.39Å.
If there were three single and three double bonds, we would expect their
lengths to be 1.54Å and 1.34Å, respectively, since these are the bond lengths in alkanes and alkenes.
The fact that the bonds in benzene seem to be intermediate between single and double bonds has received its most satisfactory explanation by the application of the theory of resonance.
This theory explains that the three electron pairs (or six electrons) enclosed in the ring are not localized or fixed at a particular position, but are free to move about within the ring. Hence, two equally reasonable structures which differ only in the location of electrons can be written for the benzene molecule:
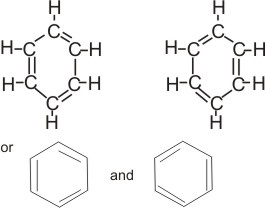
Notice that the “real” structure is neither of these. The real structure is a resonance hybrid that is intermediate in character between the two.
All six carbon-carbon bonds are equivalent. Each is more than single and less than double. That is, the real structure is a hybrid bond that cannot be satisfactorily represented on paper or with models. However, there are two methods that are used in attempting to represent the structure of benzene.
These are:
1. By writing the two contributing structures above with braces enclosing them and a two-headed arrow separating them:
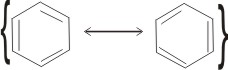
This symbolism must not be taken to mean tht molecules of two structures exist in equilibrium, but that only one kind of molecule exists: a hybrid of the two contributing structures.
2. By using a simplified symbolism. This is frequently used, and it consists of a hexagon with a circle inside:
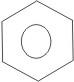
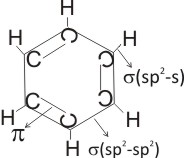
From the Kekulé structure of benzene above, it is seen that all the carbon
atoms use sp2 hybridization. Each carbon atom, using it’s sp2 hybridized orbitals forms a sigma bond with an adjacent member (i.e. sp2-sp2 ), and another sigma bond with the s orbital of hydrogen. A pi
( p) bond is formed between the unhybridized 2p orbitals.
Properties of Benzene
Physical Properties
1. Benzene is a liquid with a sweet smell
(or aroma).
2. It burns in air with a sooty flame - due to high proportion of unburnt carbon.
3. It is insoluble in water, but dissolves organic compounds.
4. It has a boiling point of 80oC.
Chemical Properties
The characteristic reactions of benzene are substitution reactions in which one or more hydrogen atoms are replaced with another atom or group of atoms.
However, addition reactions occur, but only under drastic conditions, such as high temperature and pressure and the use of catalysts. This shows that benzene is highly stable, in spite of the fact that it is highly unsaturated.
Note:
The stability of benzene is due to the fact that the delocalized electrons in the ring are not readily accessible or available for chemical reactions. Hence, addition reactions are not readily possible.
Examples of substitution reactions of benzene:
1. Nitration
Benzene reacts with a mixture of concentrated nitric and sulphuric acids at below 50oC to produce nitrobenzene.
| C6H6 +
HNO3
|
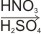 |
C6H5NO2 + H2O
|
|
|
2. Sulphonation
Benzene reacts with hot conc. H2SO4 or cold oleum to give benzenesulphonic acid.
C6H6 + H2SO4
® C6H5SO3H + H2O
3. Halogenation
In the presence of a catalyst, usually in the form of iron(III) halide, chlorine or bromine reacts with benzene.
| C6H6 + Br2
|
®
FeBr3 |
C6H5Br + HBr
|
|
|
4. Friedel Crafts reactions
Benzene reacts with either alkyl halide or an acyl halide in the presence of a Lewis acid (e.g. AlCl3) to give alkyl benzene or phenyl ketone respectively and the corresponding hydrogen halide.
| C6H6 + C2H5Br
|
anhydrous
® |
C6H5C2H5 + HBr
|
|
|
| alkylhalide
|
®
AlCl3 |
ethylbenzene
|
- this process is known as alkylation |
|
C6H6 +
CH3COCl
acylhalide
|
anhydrous
®
AlCl3 |
C6H5COCH3
phenyl ethanone
|
+ HCl |
- this process is known as acylation |
Example of addition reactions of benzene:
Hydrogenation of benzene
Benzene reacts with hydrogen in the presence of a catalyst, and at very high temperatures and pressures to form cyclohexane.
|
+ 3H2 |
Catalyst
®
high temp. and press. |
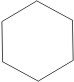 cyclohexane cyclohexane |
|
Nomenclature of
Benzene Compounds
1. The parent name of a substituted benzene is benzene. However, some common names are
accepted in the I. U. P. A. C. system of naming for certain substituted benzene.
Examples:
Note: Benzene with only one substituent does not have any isomer i.e.,
C6H5Cl 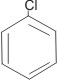
chlorobenzene
|
is the same as |
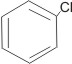
chlorobenzene |
|
|
This is because all the C-C bonds are equal in every respect.
2. If there are more than one substituent, the positions of the substituents are indicated by numbering the edges of the hexagonal shape, starting from a major substituent, or by indicating if the substituents are ortho, meta or para to the major substituent on the ring.
Position 2 is ortho(o), 3 is meta(m) and 4 is para(p) to the major substituent x.
Examples:
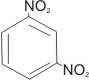
1,3-dinitrobenzene
or m-dinitrobenzene
|
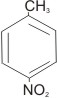
4-
nitrotoluene or p-nitrotoluene
|
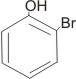
2-bromophenol
or o-bromophenol |
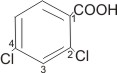
2,4-dichlorobenzoic
acid or o,p-dichlorobenzoic acid |
|
Uses of Benzene
and Its Derivatives
1. Benzene is used as solvent for organic
compounds.
2. To prepare a lot of products with important uses:
(i). Benzene hexachloride - used as
insecticides.
(ii). Phenylethene (styrene) - polymerised into polystyrene (a plastic product).
(iii). Phenol - polymerised into compounds used in dyes and as disinfectant.
(iv). Cyclohexane - polymerised to give
nylon - used in carpet.
Distinction Between Benzene and Aliphatic Hydrocarbons
Benzene, being highly unsaturated (with 6 electrons in the ring) would have been expected to give similar chemical reactions as those of the unsaturated aliphatic hydrocarbons (alkenes and alkynes), but this is not so.
As has been discussed, the reason is due to the unavailability or unaccessibility of the 6 electrons in the ring to an attacking species.
The following are therefore the differences between the reactions of benzene and those of the unsaturated aliphatic hydrocarbons:
1. The characteristic reactions of benzene are substitution (addition will only occur under drastic conditions); those of alkenes and alkynes are addition.
2. Benzene is resistant to common oxidizing agents - it gives no reaction with either bromine water or bromine vapor, or with KMnO4.
Alkenes and alkynes are readily oxidized.
3. The heat of hydrogenation of benzene is less than its theoritical value (i.e., it is less than that of 1,3,5 -hex-triene,
CH2=CH-CH=CH-CH=CH2).
This means that benzene is more stable than the unsaturated aliphatic hydrocarbons.
|