
This post focuses on acetone and hazards associated with the chemical, and gives valuable ideas on safety procedures to take when handling it.
Please, continue reading:
What is Acetone?
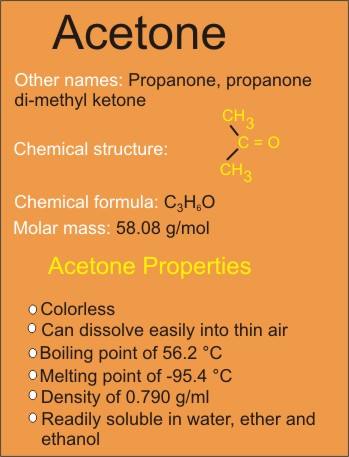
Acetone, also known as propanone is an organic compound that is represented chemically as CH3COCH3 or C3H6O. It is a colorless, highly flammable liquid.
Acetone belongs to a chemical class called ketones. It is the smallest ketone. Acetone easily mixes well with water and is a very useful solvent for different purposes.
Acetone is a powerful solvent employed for laboratory and industrial purposes; there is some use for it at home, for example erasing nail polish.
Acetone is currently derived from petrochemicals as a co-product of phenol, but there is some history of acetone being produced from the fermentation of sugar brought out from corn and other agro yields.
Acetone is a ketone, not like alcohol such as ethanol. Ketones are more oxidized than alcohols because they have less energy content (than alcohols).
Common Acetone Hazards
Acetone is not a substance that should be inhaled or swallowed. When swallowed or inhaled, it causes discomfort in the digestive system which leads to vomiting or stomach ache.
It can also lead to unconsciousness or coma and affect the menstrual circle (if the victim is a woman). Excess exposure or contact can also cause irritation in the eyes, nose and even the skin.
Acetone Safety Handling Procedures
To avoid hazards caused by acetone, there are handling procedures that you can follow to ensure safety. Some of them are as follows:
- Be sure the environment is well ventilated. Always remember acetone is inflammable. Accumulated acetone gas could easily get concentrated, which means that a single spark could result in a fire breakout. When working with the chemical, make sure you are in a very spacious and ventilated environment.
- Always be ready to fight a fire. Just in case vapor ignites, you would need to fight the fire. If it is huge fire, then you should vacate the area immediately. If it seems small, utilize dry chemical powder to put out the fire. You can make use of alcohol foam and water spray to extinguish the fire.
- No fire within the area. Acetone is extremely inflammable. Make sure there is no trace of fire or heat, e.g. smoking, soldering, welding, etc. around your surrounding while using acetone.
- Put on respirator. Make sure you are wearing a respirator in order to protect your airways. Acetone is toxic to the lungs (and the respiratory system generally), protecting your airways should be one of your top priorities when handling acetone.
Acetone Safety Storage Procedures
Acetone should be stored in a cool, dry place; for example, a well-ventilated building where chemicals are stored.
The building where chemicals are stored must be in an area where the likelihood of fire breakout is very low.
The storage of acetone is very vital since it has many uses and application in daily life.
Acetone vapours can easily be ignited by sparks or flames, therefore, a well-ventilated building for storing chemicals is highly recommended.
Acetone Safety Disposal Procedures
Acetone is meant to be disposed properly after use. If you fail to do so, it may wreak havoc on the health of people. Please follow this procedure in order to dispose it safely.
- Make use of a sealed metal or glass container to store acetone before disposal. Do not make use of plastic. Acetone could easily melt some plastics.
- Store the sealed container (glass or metal) in a dry and secluded environment away from little children, pets, or fire.
- Take the closed container (glass or metal) to the disposal facility. Be careful as you carry the container(s), so none of the containers opens up or breaks causing the acetone to leak out.
How to Handle Exposures to Acetone
Just in case you get too exposed to acetone, here are some first aid procedures you can apply immediately:
- Inhalation: If someone inhales acetone accidentally, take the person immediately to an open space where there is ventilation. Contact a doctor right away.
- Skin contact: Just in case acetone touches your skin, rinse affected part thoroughly with water. If you notice any form of irritation, contact the doctor immediately.
- Eye contact: If acetone touches the eye(s), immediately rinse the affected part with lukewarm water for about 15 to 20 minutes while holding the eyelid(s) open. Do not hesitate to flush or remove contact lens if you are wearing one. Contact a doctor if pain or irritation continues.
- Ingestion: Acetone should be used externally only. If it enters the mouth, rinse mouth with enough water. Contact a doctor immediately.
Acetone Properties (Physical and Chemical)
Physical: Acetone is a chemical that has no color and can dissolve into thin air easily. Acetone’s chemical formula has carbon atoms, three specifically (C3H6O), which makes it an organic compound. Acetone is constituted from three carbon atoms, six hydrogen atoms and one oxygen atom.
Its boiling point is known to be at 56.53oC and its melting point is exactly -95.4oC.
Acetone’s density is calculated to be at 0.790 g/cm3, and the compound dissolves when put in cold water. It is also readily soluble in ether and ethanol.
Chemical: Acetone is a compound represented chemically as C3H6O. The molecular weight is 58.08 g/mole.
Acetone Preparation
Propylene is the substance used in producing Acetone. Most of the acetone produced is made possible through the “Cumene process”.
The production process of acetone is exactly the same as that of phenol. When benzene that is usually alkylated is combined with propylene, it produces cumene. Then when cumene is mixed with oxygen, it produces acetone and phenol.
Acetone Uses
Acetone is a chemical widely used for various purposes. It is one of the major ingredients used in producing nail polish eraser, used for removing paints on nails. It is also used in making lacquers, a glossy liquid used in coating furniture or automobiles.
Conclusion
Acetone is a chemical substance we use both in the laboratory and in our homes. One has to be cautious when using it since it is toxic on the skin and the lungs when inhaled.
Make sure to follow the safety procedures highlighted above in dealing with acetone hazards.
