|
Home
Law of Conservation of Matter
The law of conservation of matter states that matter is neither created nor destroyed in the course of a chemical reaction. The law states the fact that the total masses of the products from a chemical reaction exactly equal those of the reactants. The law can be illustrated by the experiment below:
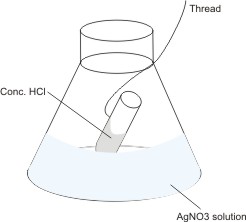
The mass of the set-up as shown is measured and recorded. By the thread, the HCl solution in the test tube is mixed with the silver trioxonitrate(V) solution in the flask. A reaction
occurs, leading to the production of white precipitate (AgCl). The mass of the set-up is measured again. It is found that
in spite of the formation of a solid substance, the mass remains the same.
Matter is neither lost nor gained during chemical reactions, but only change from one form to another because, according to Dalton’s atomic theory, the atoms in reaction undergo reconstitution during chemical reactions to form the products, and not that new atoms are formed, or that some get destroyed.
The above experiment can be performed with appropriate solutions of other substances. Example: barium chloride and sodium tetraoxosulphate(VI); barium chloride and dilute tetraoxosulphate(VI) acid; lead trioxonitrate(V) and potassium iodide; calcium trioxonitrate(V) and dilute tetraoxosulphate(VI) acid.
A more accurate experiment to illustrate the law of conservation of matter was done by a scientist called Landolt in 1908. It was done in the apparatus show below:
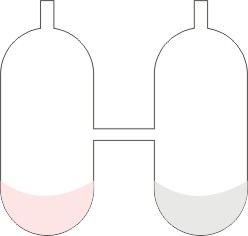
In the separate arm of the tube are put sodium chloride and silver nitrate solutions. The tube and its contents were weighed. The tube was tilted to enable the content mix-up and react. It was then cooled, and reweighed. It was found that the total weight of the apparatus and the substances in it remained constant before and after reaction.
|