|
Home
Electrolytic Cell | Electrochemical Cell
An electrolytic cell is a type of electrochemical
cell that undergoes both oxidation and reduction reactions (redox
reaction) when electrical energy is passed into it.
Note: electrochemical cells are systems that can
either generate electricity from chemical substances called
electrolytes, or use
electrical energy from an external source to facilitate chemical reactions,
leading to the decomposition of the electrolyte.
Electrochemical cells consist of two half cells,
each of which consisting of an
electrode and an electrolyte. Some
electrochemical cells consist of the same electrolyte in the two half cells
while others have different electrolytes.
Examples of electrochemical cells include Voltaic or
Galvanic cell, which generates electricity from chemical substances; and
electrolytic cell, which uses external electrical energy source to cause the
decomposition of the electrolyte.
An electrolytic cell is obtained by connecting a source of direct current, DC into an electrolyte through
electrodes. The direct current is provided by batteries whose positive end is connected by wire to the positive electrode (called anode) and negative end connected to the negative electrode (called cathode) as shown below:
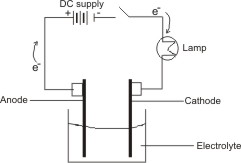
When the switch is on, electrons flow from
the negative end of the batteries into the
cathode. The concentration of electrons on
the cathode causes the positive ions in
solution to migrate to the cathode, while
negative ions migrate to the anode.
At the
cathode, one of the positive ions picks up
electrons and become reduced, while at the
anode, one of the negative ions loses the
same number of electrons picked up at the
cathode and become oxidized. The lost
electrons are taking away through the wire,
back to the batteries.
The bulb lights up,
indicating that the circuit is complete, which
means that the solution is a conductor of
electricity. The process continues, with
gradual reduction in the intensity of the light
due to the decomposition and loss of the
electrolyte.
Notice that conduction of electricity is due to the migration of opposite ions
to the electrodes.
The above set-up can be used to determine whether the solution of a substance is an electrolyte or not (solutions of non-electrolytes will not produce light in the bulb).
It can also be used to differentiate strong electrolytes from weak electrolytes (strong electrolytes will produce bright light, while weak electrolytes will produce dull light).
The chemical reactions that took place at both
electrodes needed external electrical power to be applied before they would
occur. This means they are non-spontaneous reactions.
Examples of Electrolytic Cell
Electrolysis of Dilute H2SO4,
Also called Electrolysis of Water
Electrolysis of dilute H2SO4
is also called electrolysis of water because hydrogen and oxygen, in the ratio of 2:1 are the products of the
electrolysis.
The following ions are present in solution:
H+ + OH- from water and
2H+ + SO42- from H2SO4
The positive ions, .i.e., H+ migrate to the
cathode (the negative plate) while the negative ions, OH- and SO42- migrate to the anode (the positive plate). Both cathode and anode are platinum foil.
At the Cathode
H+ that have migrated here gain electrons i.e. 2H+(aq) + 2e-(aq)
→ H2(g)
Note: Due to the fact that H+ is discharged here, and that SO42- had migrated from here to the anode, there is going to be a decrease in acidity (i.e. less H2SO4).
1 mole of hydrogen = 1 vol. is produced here.
At the Anode
Of OH- and SO42- that migrated here, OH- is discharged (due to its lower position in the
electrochemical series).
2OH- → H2O(l) + ½ O2(g) + 2e-(aq)
(the number of electrons lost here must be same as those acquired at the cathode)
Note: The ionic equilibrium of water is disturbed by the discharge of OH-, naturally, the system will ionize more water, thereby causing excess of H+ to be produced. Hence H+ combine with SO42- that were not discharged to make the anode acidic (H2SO4) – acidity increases.
By the above equation of the discharge of OH-, it is shown that ½ mole or ½ vol. of oxygen is produced here.
The mole ratio, or the ratio of the volumes hydrogen to oxygen produced at the cathode and at the anode is 2 : 1
The total acidity in the system (i.e., at anode and cathode together) remains constant – because the ions discharged are H+ and OH- from water.
Electrolysis of CuSO4 Solution
The ions present in solution are:
H+ + OH- from H2O;
Cu2+ + SO42- from CuSO4
The positive ions, i.e. H+ and Cu2+ migrate from the anode to the cathode while the negative ions OH- and SO42- migrate from the cathode to the anode.
At the Cathode
Cu2+ is discharged in preference to H+ (Cu2+ is lower than H+ in the electrochemical series).
Cu2+(aq) + 2e-(aq) → Cu(s)
Note: copper is deposited as a brown layer
At the Anode
The nature of anode will determine which ion is discharged.
If either platinum or carbon electrode is used, then the OH- is discharged instead of SO42-, i.e.
2OH-(aq) → H2O(l) + ½ O2(g)
Note: oxygen gas is given off, the anode becomes acidic due to the concentration of undischarged SO42- and excess H+ produced from ionization of more water (in order to replace the OH- that was discharged).
Hence, the whole solution becomes acidic with the H2SO4.
If the anode is a copper metal non of OH- or SO42- is discharged, rather, the copper metal passes into the solution as Cu2+,
I.e. Cu(s) → Cu2+(aq) + 2e-(aq)
Note:
If copper anode is used, the total concentration of the solution in SO42- (which is not discharged) and Cu2+ is constant. This implies that the electrolysis is simply a transfer of copper from the anode to cathode. The mass of the copper anode decreases with time.
If platinum or carbon electrodes are used, the color of the solution (blue Cu2+) will be observed to fade as copper is depositing at the cathode. However, with copper electrodes, the color remain constant – because as Cu2+ leaves the solution and become deposited as Cu(s) at the cathode, more of Cu2+ gets into solution from the decomposition of the copper anode.
Electrolysis of
Conc. NaCl Solution (Brine)
The ions present are:
H+ + OH- from H2O;
Na+ + Cl- from NaCl
At the Cathode
Both H+ and Na+ migrate to the cathode. But H+ being lower in the electrochemical series is discharged in preference to Na+
2H+(aq) + 2e-(aq) → H2(g)
The ionic equilibrium of water is disturbed by the discharge of H+, hence more water ionize, producing excess of OH-, which combine with the undischarged Na+ to make the cathode alkaline (NaOH).
Note: 1 mole or 1 vol. of hydrogen gas is produced here.
At the Anode
Both OH- and Cl- migrate here from the cathode. Cl- ion is discharged in preference to OH- ion because it is present in much greater concentration than OH-.
2Cl-(aq) → Cl2(g) + 2e-(aq)
Note: 1 mole or 1 vol of Cl2 is produced, although in practice, it is less than this because some oxygen is also always produced from partial discharge of OH-.
And if the solution is much diluted, OH- will be discharged completely in preference to Cl-
Carbon anode must be used because chlorine, which is produced here attacks platinum but is resistant to carbon.
The overall nature of the solution is alkaline (presence of NaOH).
Electrolysis of Fused NaCl
The ions present are: Na+ + Cl- (water is not present to provide the ions: H+ + OH-)
At the Cathode
2Na+ gains electrons and become reduced
to the metal: 2Na+(aq) + 2e-(aq) → 2Na(s)
At the Anode
Cl- gives off electrons and becomes oxidized: 2Cl-(aq)
→ Cl2(g) + 2e-(aq)
This method is used for the commercial preparation of sodium metal and chlorine gas.
Electrolysis of CuCl2 Solution
The ions present are:
H+ + OH- from water;
Cu2+ + Cl- from CuCl2
At the Cathode
Cu2+, being lower in the electrochemical series is discharged in preference to H+
I.e.,
Cu2+(aq) + 2e-(aq) → Cu(s)
Note: copper metal is deposited as brown layer
at the cathode.
At the Anode
The nature of anode used will determine
the ions to be discharged.
If carbon anode is used Cl- will be
discharged in preference to OH-
because it is in greater concentration.
I.e. 2Cl-(aq) → Cl2(g) + 2e-(aq)
Chlorine gas is produced.
But if copper metal is used
as anode, non of Cl- or OH- is
discharged, but the copper anode
decomposes into solution
Cu(s) → Cu2+(aq) + 2e-(aq)
The total concentration of the
solution (i.e. Cu2+ and Cl- will
remain the same) if copper
anode is used.
Generally, reduction occurs at the cathode while oxidation occurs at the anode.
Electrolysis is not a spontaneous reaction as electricity is needed to drive the reactions at both electrodes, hence work is done on the system.
|

