Alkenes, like the
alkanes can
show structural isomerism. This is possible due to changes in the position of
the double bond or in the position of their substituents. E.g.,
|
1. CH3 - CH2
- CH = CH2
1 -butene (C4H8) |
and |
CH3
- CH = CH - CH3
2- butene (C4H8) |
|
|
|
2.
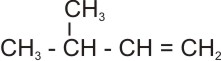
3- methyl -1-butene (C5H10)
|
and |
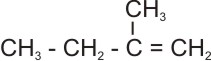
2-
methyl-1-butene (C5H10) |
|
|
Geometrical isomerism
Alkenes can also show geometrical
isomerism. This occurs due to the restriction to rotation about the C = C bond
(the double bond is rigid).
Hence, the two groups attached to each carbon in the
double bond are held rigidly in a specific orientation in space. Therefore,
geometrical isomers have different orientations in space (each orientation
appears distinct, and thus shows difference in both physical and chemical
properties from the other), but have the same molecular formula and structure.
When the same groups (or priority groups) are on the same side of the double
bond, the geometrical isomer is regarded as a cis.
Example,
|
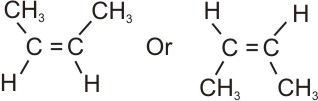
Cis Butene
(the methyl groups are on the same side)
|
|
|
|
|
When they are on opposite sides, the isomer is a Trans.
Example,
|
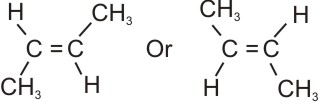
Trans-butene
|
|
|
|
|
Further examples:
|
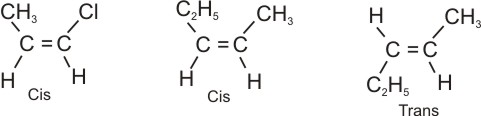
1-chloro-1-propene
2-pentene |
|
|
|
|
Note:
* Geometrical isomers (cis and trans)
are different from each other in both physical and chemical properties. This is
due to the difference in their orientation in space, which is due to the
rigidity of the double bond.
* The Trans isomers are usually more stable than their
Cis counterpart.
* Not all alkenes show geometrical isomerism.
Geometrical isomerism is not possible if two priority groups are attached to one
carbon in the double bond.
* For the fact that alkenes show both structural and
geometrical isomerism, a particular alkene has more isomers than it
corresponding alkane.
Examples of compounds which can not show geometrical
isomerism:
|
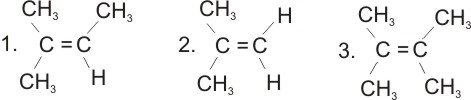
2-methylbut-2-ene
2-methylprop-1-ene
2,3-dimethylbut-2-ene |
|
|
|
|
|
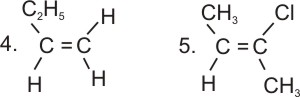
But-1-ene
2-chloro-2-butene |
|
|
|
|
Related:
Alkenes
Polymerization Reactions of Alkenes

