|
Home
Alkyl Halides
What is Alkyl halide?
Alkyl halides are halogen substituted products of
alkanes. They have the general formula CnH2n+1X or RX. Where R represents the alkyl group, and X the halogen (usually any of Cl, Br, and I).
Nomenclature of Alkyl Halides
They are named by adding the prefix chloro-, bromo-, or iodo- to the parent hydrocarbon, and indicating the position of attachment of the halogen atom.
E.g.
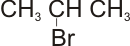
(2 - bromo propane) |
|
|
|
|
Classification of Alkyl Halides
Alkyl halides are classified in a similar way as alkanols. i.e., primary (1o), Secondary (2o) and tertiary (3o) - depending on the number of alkyl groups attached to the carbon bearing the halide.
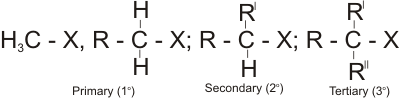
Preparation of Alkyl Halides
1. From alkanols - phosphorus halides, hydrogen halides or thionyl chlorides with alkanols give alkyl halides.
E.g.
CH3CH2OH + HBr
®
CH3CH2Br + H2O
3CH3CH2OH + PBr3
®
3CH3CH2Br + H3PO3
CH3CH2OH + PCl5
®
CH3CH2Cl + POCl3 + HCl
CH3CH2OH + SOCl2
®
CH3CH2Cl + SO2 + HCl
2. From Alkenes - hydrogen halides react with ethene by addition reaction to form alkyl halides.
CH3CH = CH2 + HBr
®
CH3CH Br CH3
(2 - bromo propane)
Note:
* The carbon having the lower number of hydrogen atom bears the electronegative element Br -, while the other
carbon bears the hydrogen.
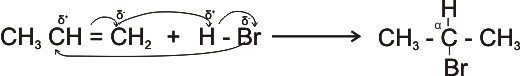
This pattern of addition of hydrogen halide to alkenes is known as the
Markonikov’s rule.
*d+ - partial positive charge;
d- - partial negative charge.
*The carbon bearing the halogen is
designated as the
a - carbon.
3. By halogenation of hydrocarbons - halogens (e.g. chlorine and bromine) react with alkanes in the presence of light to give substituted products.
C2H6(g) + Cl2(g) Light → C2H5Cl(g) + HCl(g)
Properties of Alkyl Halides
Physical Properties
1. Few are gases at room temperature, e.g., bromoethane; few are liquids; while most are solids.
2. They are insoluble in water and soluble in organic solvent.
3. Their boiling points decrease with branches, i.e., the branched isomers are of lower boiling points than their straight chain isomers. Therefore, tertiary alkyl halides are of lower boiling points than their secondary isomers; and secondary isomers are of lower boiling points than their primary isomers.
Chemical Properties
1. Nucleophillic substitution
In alkyl halides, the C-X bonding electrons are attracted more to the halogen (halogens are highly electronegative). The carbon atom becomes partially positively charged (d+) and becomes easily attacked by a nucleophile.
A nucleophile is a negatively charged species which is looking for a positive centre to attach. Examples of nucleophiles are OH-, NO2-, CH3CH2O-, CN-, NH3 and Cl-.
The reaction between a positive carbon and a nucleophile, with the displacement of a negative species, e.g., halogen element is called nucleophillic substitution reaction.
Examples:
(a).
Reactions between alkyl halides and aqueous NaOH produces alkanols.
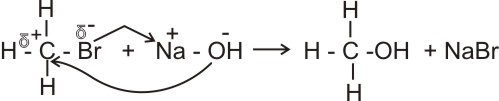
Bromomethane
Methanol
|
|
|
|
|
(b). Alkyl halides react with alkoxide ions in alkanolic solutions to give ethers (the williamson’s ether synthesis).
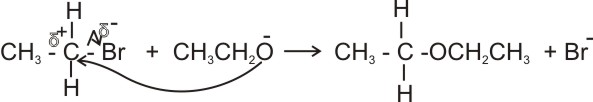
Ethoxide ion
Diethylether
|
|
|
|
|
(c). Cyanide ions in aqueous alkanolic solutions react with alkyl halides to displace halogens and produce nitriles.
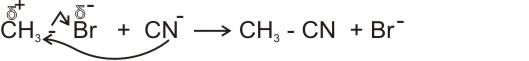
ethanenitrile
|
|
|
|
|
(d). Ammonia and amines are nucleophiles because they have lone pair of electrons on their nitrogen atoms. They therefore react with alkyl halides to give substituted ammonium salts.
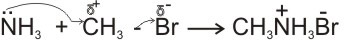
methyl ammonium bromide
|
|
|
|
|
2. Elimination reactions
Elimination reactions may accompany substitution reactions depending on the structure of alkyl halides, temperature, strength of alkalis and polarity of the solvent used.
High concentration of strong alkali, high temperatures, low polarity of the solvent and the use of large molecular alkyl halides will all favor elimination reaction.
For example, bromoethane is attacked by hot alkanolic solution of conc. KOH to produce a high yield of ethene together with low quantity of ethanol (from substitution reaction).
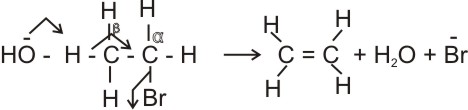
Note:
* Hydrogen is lost from
b carbon.
* With methyl halide, only substitution
reaction is possible, i.e., only methanol is produced.
3. Reaction with metals
(a). With sodium or potassium - alkyl halides react with sodium or potassium in dry ether solution to give alkanes (this reaction is known as wurtz reaction).
| 2CH3CH2I
+ 2Na
®
|
CH3CH2CH2CH3 + 2NaI |
|
|
|
(b). With magnesium - alkyl halides react with magnesium in anhydrous ether to give compounds called Grignard reagents (these compounds are very useful in the production of many different compounds).
| CH3CH2Br
+ Mg
|
+ dry ether
®
|
3CH2MgBr |
|
|
| |
|
Ethyl magnesium bromide
(Grignard reagent) |
|
|
(c). With zinc/copper couple - alkyl halides are reduced by zinc/copper couple (i.e. zinc powder coated with copper) in ethanol.
The product is the corresponding alkane.
| 2CH3CH2Br
+ Zn/Cu + 2CH3CH2OH
® |
2C2H6 + (CH3CH2O)2 + Zn2+ + 2Br- |
|
|
|
Uses of Alkyl Halides
1. As solvent for organic solids.
2. For the preparation of many organic compounds such as alkanols.
3. As refrigerants.
4. As pesticides and fumigating agents.
|

